Related Research Articles

Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation. Dry distillation is the heating of solid materials to produce gaseous products. Dry distillation may involve chemical changes such as destructive distillation or cracking and is not discussed under this article. Distillation may result in essentially complete separation, or it may be a partial separation that increases the concentration of selected components in the mixture. In either case, the process exploits differences in the relative volatility of the mixture's components. In industrial applications, distillation is a unit operation of practically universal importance, but it is a physical separation process, not a chemical reaction.
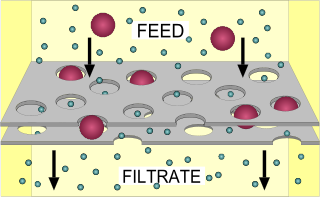
Filtration is a physical separation process that separates solid matter and fluid from a mixture using a filter medium that has a complex structure through which only the fluid can pass. Solid particles that cannot pass through the filter medium are described as oversize and the fluid that passes through is called the filtrate. Oversize particles may form a filter cake on top of the filter and may also block the filter lattice, preventing the fluid phase from crossing the filter, known as blinding. The size of the largest particles that can successfully pass through a filter is called the effective pore size of that filter. The separation of solid and fluid is imperfect; solids will be contaminated with some fluid and filtrate will contain fine particles. Filtration occurs both in nature and in engineered systems; there are biological, geological, and industrial forms.

In the physical sciences, a phase is a region of space, throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, magnetization and chemical composition. A simple description is that a phase is a region of material that is chemically uniform, physically distinct, and (often) mechanically separable. In a system consisting of ice and water in a glass jar, the ice cubes are one phase, the water is a second phase, and the humid air is a third phase over the ice and water. The glass of the jar is another separate phase.
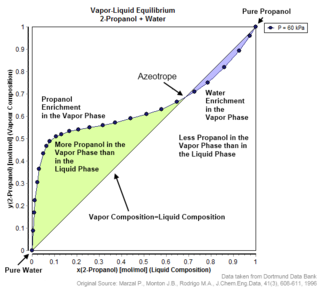
An azeotrope or a constant heating point mixture is a mixture of two or more liquids whose proportions cannot be altered or changed by simple distillation. This happens when an azeotrope is boiled, the vapour has the same proportions of constituents as the unboiled mixture. Because their composition is unchanged by distillation, azeotropes are also called constant boiling point mixtures.

In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.

High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify each component in a mixture. It relies on pumps to pass a pressurized liquid solvent containing the sample mixture through a column filled with a solid adsorbent material. Each component in the sample interacts slightly differently with the adsorbent material, causing different flow rates for the different components and leading to the separation of the components as they flow out of the column.
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation to fractionate. Generally the component parts have boiling points that differ by less than 25 °C (45 °F) from each other under a pressure of one atmosphere. If the difference in boiling points is greater than 25 °C, a simple distillation is typically used. It is used to refine crude oil.
Pervaporation is a processing method for the separation of mixtures of liquids by partial vaporization through a non-porous or porous membrane.

A fractionating column or fractional column is an essential item used in the distillation of liquid mixtures to separate the mixture into its component parts, or fractions, based on the differences in volatilities. Fractionating columns are used in small scale laboratory distillations as well as large scale industrial distillations.

Fractional freezing is a process used in process engineering and chemistry to separate substances with different melting points. It can be done by partial melting of a solid, for example in zone refining of silicon or metals, or by partial crystallization of a liquid, as in freeze distillation, also called normal freezing or progressive freezing. The initial sample is thus fractionated.

In chemical engineering and related fields, a unit operation is a basic step in a process. Unit operations involve a physical change or chemical transformation such as separation, crystallization, evaporation, filtration, polymerization, isomerization, and other reactions. For example, in milk processing, the following unit operations are involved: homogenization, pasteurization, and packaging. These unit operations are connected to create the overall process. A process may require many unit operations to obtain the desired product from the starting materials, or feedstocks.
The transfer is driven by chemical potential, i.e. once the transfer is complete, the overall system of chemical components that make up the solutes and the solvents are in a more stable configuration. The solvent that is enriched in solute(s) is called extract. The feed solution that is depleted in solute(s) is called the raffinate. LLE is a basic technique in chemical laboratories, where it is performed using a variety of apparatus, from separatory funnels to countercurrent distribution equipment called as mixer settlers. This type of process is commonly performed after a chemical reaction as part of the work-up, often including an acidic work-up.

Continuous distillation, a form of distillation, is an ongoing separation in which a mixture is continuously fed into the process and separated fractions are removed continuously as output streams. Distillation is the separation or partial separation of a liquid feed mixture into components or fractions by selective boiling and condensation. The process produces at least two output fractions. These fractions include at least one volatile distillate fraction, which has boiled and been separately captured as a vapor condensed to a liquid, and practically always a bottoms fraction, which is the least volatile residue that has not been separately captured as a condensed vapor.
A zeotropicmixture, or non-azeotropic mixture, is a mixture with liquid components that have different boiling points. For example, nitrogen, methane, ethane, propane, and isobutane constitute a zeotropic mixture. Individual substances within the mixture do not evaporate or condense at the same temperature as one substance. In other words, the mixture has a temperature glide, as the phase change occurs in a temperature range of about four to seven degrees Celsius, rather than at a constant temperature. On temperature-composition graphs, this temperature glide can be seen as the temperature difference between the bubble point and dew point. For zeotropic mixtures, the temperatures on the bubble (boiling) curve are between the individual component's boiling temperatures. When a zeotropic mixture is boiled or condensed, the composition of the liquid and the vapor changes according to the mixtures's temperature-composition diagram.
A theoretical plate in many separation processes is a hypothetical zone or stage in which two phases, such as the liquid and vapor phases of a substance, establish an equilibrium with each other. Such equilibrium stages may also be referred to as an equilibrium stage, ideal stage, or a theoretical tray. The performance of many separation processes depends on having series of equilibrium stages and is enhanced by providing more such stages. In other words, having more theoretical plates increases the efficiency of the separation process be it either a distillation, absorption, chromatographic, adsorption or similar process.
This glossary of chemistry terms is a list of terms and definitions relevant to chemistry, including chemical laws, diagrams and formulae, laboratory tools, glassware, and equipment. Chemistry is a physical science concerned with the composition, structure, and properties of matter, as well as the changes it undergoes during chemical reactions; it features an extensive vocabulary and a significant amount of jargon.

A centrifugal extractor—also known as a centrifugal contactor or annular centrifugal contactor—uses the rotation of the rotor inside a centrifuge to mix two immiscible liquids outside the rotor and to separate the liquids in the field of gravity inside the rotor. This way, a centrifugal extractor generates a continuous extraction from one liquid phase into another liquid phase.
Solvent impregnated resins (SIRs) are commercially available (macro)porous resins impregnated with a solvent/an extractant. In this approach, a liquid extractant is contained within the pores of (adsorption) particles. Usually, the extractant is an organic liquid. Its purpose is to extract one or more dissolved components from a surrounding aqueous environment. The basic principle combines adsorption, chromatography and liquid-liquid extraction.
A separation process is a method that converts a mixture or solution of chemical substances into two or more distinct product mixtures. In other words, it's a scientific process of distinguishing to two or more substance in order to obtain purity. At least one product mixture of the separation is enriched in one or more of the source mixture's constituents. In some cases, a separation may fully divide the mixture into pure constituents. Separations exploit differences in chemical properties or physical properties between the constituents of a mixture.
References
- 1 2 Bosch, André B. de Haan, Hans (2013). Industrial separation processes: fundamentals. Berlin: De Gruyter. ISBN 978-3-11-030669-9.