Related Research Articles

The vomeronasal organ (VNO), or Jacobson's organ, is the paired auxiliary olfactory (smell) sense organ located in the soft tissue of the nasal septum, in the nasal cavity just above the roof of the mouth in various tetrapods. The name is derived from the fact that it lies adjacent to the unpaired vomer bone in the nasal septum. It is present and functional in all snakes and lizards, and in many mammals, including cats, dogs, cattle, pigs, and some primates. Some humans may have physical remnants of a VNO, but it is vestigial and non-functional.

An olfactory receptor neuron (ORN), also called an olfactory sensory neuron (OSN), is a sensory neuron within the olfactory system.

Olfactory receptors (ORs), also known as odorant receptors, are chemoreceptors expressed in the cell membranes of olfactory receptor neurons and are responsible for the detection of odorants which give rise to the sense of smell. Activated olfactory receptors trigger nerve impulses which transmit information about odor to the brain. These receptors are members of the class A rhodopsin-like family of G protein-coupled receptors (GPCRs). The olfactory receptors form a multigene family consisting of around 400 genes in humans and 1400 genes in mice.
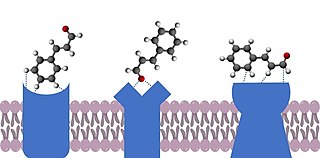
The docking theory of olfaction proposes that the smell of an odorant molecule is due to a range of weak non-covalent interactions between the odorant [a ligand] and one or more G protein-coupled odorant receptors. These include intermolecular forces, such as dipole-dipole and Van der Waals interactions, as well as hydrogen bonding. More specific proposed interactions include metal-ion, ion-ion, cation-pi and pi-stacking. Interactions can be influenced by the hydrophobic effect. Conformational changes can also have a significant impact on interactions with receptors, as ligands have been shown to interact with ligands without being in their conformation of lowest energy.
The antennal lobe is the primary olfactory brain area in insects. The antennal lobe is a sphere-shaped deutocerebral neuropil in the brain that receives input from the olfactory sensory neurons in the antennae and mouthparts. Functionally, it shares some similarities with the olfactory bulb in vertebrates. The anatomy and physiology function of the insect brain can be studied by dissecting open the insect brain and imaging or carrying out in vivo electrophysiological recordings from it.
Odorant-binding proteins (OBPs) are small soluble proteins secreted by auxiliary cells surrounding olfactory receptor neurons, including the nasal mucus of many vertebrate species and in the sensillar lymph of chemosensory sensilla of insects. OBPs are characterized by a specific protein domain that comprises six α-helices joined by three disulfide bonds. Although the function of the OBPs as a whole is not well established, it is believed that they act as odorant transporters, delivering the odorant molecules to olfactory receptors in the cell membrane of sensory neurons.
Olfactory receptor 1D2 is a protein that in humans is encoded by the OR1D2 gene.

Slit homolog 2 protein is a protein that in humans is encoded by the SLIT2 gene.

Olfactory receptor 1G1 is a protein that in humans is encoded by the OR1G1 gene.

Olfactory receptor 1A2 is a protein that in humans is encoded by the OR1A2 gene.

Olfactory receptor 2W1 is a protein that in humans is encoded by the OR2W1 gene.

Olfactory receptor 7D4 is a protein that in humans is encoded by the OR7D4 gene.

Olfactory receptor 13H1 is a protein that in humans is encoded by the OR13H1 gene.

The sense of smell, or olfaction, is the special sense through which smells are perceived. The sense of smell has many functions, including detecting desirable foods, hazards, and pheromones, and plays a role in taste.

Leslie Birgit Vosshall is an American neurobiologist and currently a Howard Hughes Medical Institute (HHMI) investigator and the Robin Chemers Neustein Professor of Neurogenetics and Behavior at The Rockefeller University. In 2022 she was appointed Chief Scientific Officer and vice president of HHMI. She is also the director of the Kavli Neural Systems Institute at The Rockefeller University. Vosshall, a member of the National Academy of Sciences, is known for her contributions to the field of olfaction, particularly for the discovery and subsequent characterization of the insect olfactory receptor family, and the genetic basis of chemosensory behavior in mosquitoes. She has also extended her research into the study of human olfaction, revealing parts of human genetic olfactory architecture, and finding variations in odorant receptors that determine individuals’ abilities to detect odors.

Bill S. Hansson is a Swedish neuroethologist. From June 2014 until June 2020, he was vice president of the Max Planck Society.
John R. Carlson is an American biologist and professor. He currently holds the Eugene Higgins Professor of Molecular, Cellular, and Developmental Biology at Yale University.
Or83b, also known as Orco, is an odorant receptor and the corresponding gene that encodes it. The odorant receptor Or83b is not exclusively expressed in insects. Though its actual function is still a mystery, the broadly expressed Or83b has been conserved across highly divergent insect populations across 250 million years of evolution.

Insect olfaction refers to the function of chemical receptors that enable insects to detect and identify volatile compounds for foraging, predator avoidance, finding mating partners and locating oviposition habitats. Thus, it is the most important sensation for insects. Most important insect behaviors must be timed perfectly which is dependent on what they smell and when they smell it. For example, olfaction is essential for locating host plants and hunting prey in many species of insects, such as the moth Deilephila elpenor and the wasp Polybia sericea, respectively.

Vanessa Julia Ruta is an American neuroscientist known for her work on the structure and function of chemosensory circuits underlying innate and learned behaviors in the fly Drosophila melanogaster. She is the Gabrielle H. Reem and Herbert J. Kayden Associate Professor and Head of the Laboratory of Neurophysiology and Behavior at The Rockefeller University and, as of 2021, an Investigator of the Howard Hughes Medical Institute.
References
- ↑ Kaupp UB (March 2010). "Olfactory signalling in vertebrates and insects: differences and commonalities". Nat. Rev. Neurosci. 11 (3): 188–200. doi:10.1038/nrn2789. hdl: 11858/00-001M-0000-0028-6203-9 . PMID 20145624. S2CID 1423273.
- ↑ Wicher D, Schäfer R, Bauernfeind R, Stensmyr MC, Heller R, Heinemann SH, Hansson BS (April 2008). "Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels". Nature. 452 (7190): 1007–11. Bibcode:2008Natur.452.1007W. doi:10.1038/nature06861. PMID 18408711. S2CID 4418144.
- ↑ Sato K, Pellegrino M, Nakagawa T, Nakagawa T, Vosshall LB, Touhara K (April 2008). "Insect olfactory receptors are heteromeric ligand-gated ion channels". Nature. 452 (7190): 1002–6. Bibcode:2008Natur.452.1002S. doi:10.1038/nature06850. PMID 18408712. S2CID 4413766.
- ↑ Katritch V, Cherezov V, Stevens RC (2013). "Structure-function of the G protein-coupled receptor superfamily". Annu. Rev. Pharmacol. Toxicol. 53: 531–56. doi:10.1146/annurev-pharmtox-032112-135923. PMC 3540149 . PMID 23140243.
- ↑ Ioannidis P, Simao FA, Waterhouse RM, Manni M, Seppey M, Robertson HM, Misof B, Niehuis O, Zdobnov EM (February 2017). "Genomic Features of the Damselfly Calopteryx splendens Representing a Sister Clade to Most Insect Orders". Genome Biol Evol. 9 (2): 415–430. doi:10.1093/gbe/evx006. PMC 5381652 . PMID 28137743.
- ↑ Clark AG, Eisen MB, Smith DR, Bergman CM, Oliver B, Markow TA, Kaufman TC, Kellis M, Gelbart W, et al. (November 2007). "Evolution of genes and genomes on the Drosophila phylogeny". Nature. 450 (7167): 203–18. Bibcode:2007Natur.450..203C. doi: 10.1038/nature06341 . PMID 17994087. S2CID 2416812.
- ↑ Hansson BS, Stensmyr MC (December 2011). "Evolution of insect olfaction". Neuron. 72 (5): 698–711. doi: 10.1016/j.neuron.2011.11.003 . PMID 22153368.
- ↑ Venthur H, Zhou JJ (2018). "Odorant Receptors and Odorant-Binding Proteins as Insect Pest Control Targets: A Comparative Analysis". Front Physiol. 9: 1163. doi: 10.3389/fphys.2018.01163 . PMC 6117247 . PMID 30197600.