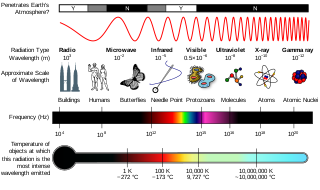
The electromagnetic spectrum is the full range of electromagnetic radiation, organized by frequency or wavelength. The spectrum is divided into separate bands, with different names for the electromagnetic waves within each band. From low to high frequency these are: radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays. The electromagnetic waves in each of these bands have different characteristics, such as how they are produced, how they interact with matter, and their practical applications.

A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word laser is an anacronym that originated as an acronym for light amplification by stimulated emission of radiation. The first laser was built in 1960 by Theodore Maiman at Hughes Research Laboratories, based on theoretical work by Charles H. Townes and Arthur Leonard Schawlow.

The photoelectric effect is the emission of electrons from a material caused by electromagnetic radiation such as ultraviolet light. Electrons emitted in this manner are called photoelectrons. The phenomenon is studied in condensed matter physics, solid state, and quantum chemistry to draw inferences about the properties of atoms, molecules and solids. The effect has found use in electronic devices specialized for light detection and precisely timed electron emission.
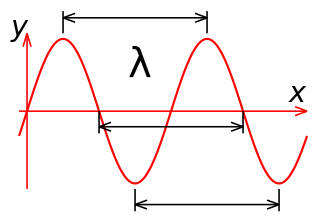
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats. In other words, it is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, troughs, or zero crossings. Wavelength is a characteristic of both traveling waves and standing waves, as well as other spatial wave patterns. The inverse of the wavelength is called the spatial frequency. Wavelength is commonly designated by the Greek letter lambda (λ). The term "wavelength" is also sometimes applied to modulated waves, and to the sinusoidal envelopes of modulated waves or waves formed by interference of several sinusoids.
In physics, attenuation is the gradual loss of flux intensity through a medium. For instance, dark glasses attenuate sunlight, lead attenuates X-rays, and water and air attenuate both light and sound at variable attenuation rates.

An optical amplifier is a device that amplifies an optical signal directly, without the need to first convert it to an electrical signal. An optical amplifier may be thought of as a laser without an optical cavity, or one in which feedback from the cavity is suppressed. Optical amplifiers are important in optical communication and laser physics. They are used as optical repeaters in the long distance fiber-optic cables which carry much of the world's telecommunication links.
Atomic, molecular, and optical physics (AMO) is the study of matter–matter and light–matter interactions, at the scale of one or a few atoms and energy scales around several electron volts. The three areas are closely interrelated. AMO theory includes classical, semi-classical and quantum treatments. Typically, the theory and applications of emission, absorption, scattering of electromagnetic radiation (light) from excited atoms and molecules, analysis of spectroscopy, generation of lasers and masers, and the optical properties of matter in general, fall into these categories.
A phonon is a collective excitation in a periodic, elastic arrangement of atoms or molecules in condensed matter, specifically in solids and some liquids. A type of quasiparticle in physics, a phonon is an excited state in the quantum mechanical quantization of the modes of vibrations for elastic structures of interacting particles. Phonons can be thought of as quantized sound waves, similar to photons as quantized light waves.

A laser diode is a semiconductor device similar to a light-emitting diode in which a diode pumped directly with electrical current can create lasing conditions at the diode's junction.

In physics, a plasmon is a quantum of plasma oscillation. Just as light consists of photons, the plasma oscillation consists of plasmons. The plasmon can be considered as a quasiparticle since it arises from the quantization of plasma oscillations, just like phonons are quantizations of mechanical vibrations. Thus, plasmons are collective oscillations of the free electron gas density. For example, at optical frequencies, plasmons can couple with a photon to create another quasiparticle called a plasmon polariton.

The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to electrons making a transition from a high energy state to a lower energy state. The photon energy of the emitted photons is equal to the energy difference between the two states. There are many possible electron transitions for each atom, and each transition has a specific energy difference. This collection of different transitions, leading to different radiated wavelengths, make up an emission spectrum. Each element's emission spectrum is unique. Therefore, spectroscopy can be used to identify elements in matter of unknown composition. Similarly, the emission spectra of molecules can be used in chemical analysis of substances.

In the field of optics, transparency is the physical property of allowing light to pass through the material without appreciable scattering of light. On a macroscopic scale, the photons can be said to follow Snell's law. Translucency allows light to pass through, but does not necessarily follow Snell's law; the photons can be scattered at either of the two interfaces, or internally, where there is a change in index of refraction. In other words, a translucent material is made up of components with different indices of refraction. A transparent material is made up of components with a uniform index of refraction. Transparent materials appear clear, with the overall appearance of one color, or any combination leading up to a brilliant spectrum of every color. The opposite property of translucency is opacity. Other categories of visual appearance, related to the perception of regular or diffuse reflection and transmission of light, have been organized under the concept of cesia in an order system with three variables, including transparency, translucency and opacity among the involved aspects.

Hydrogen-alpha, typically shortened to H-alpha or Hα, is a deep-red visible spectral line of the hydrogen atom with a wavelength of 656.28 nm in air and 656.46 nm in vacuum. It is the first spectral line in the Balmer series and is emitted when an electron falls from a hydrogen atom's third- to second-lowest energy level. H-alpha has applications in astronomy where its emission can be observed from emission nebulae and from features in the Sun's atmosphere, including solar prominences and the chromosphere.
In physics, atomic spectroscopy is the study of the electromagnetic radiation absorbed and emitted by atoms. Since unique elements have unique emission spectra, atomic spectroscopy is applied for determination of elemental compositions. It can be divided by atomization source or by the type of spectroscopy used. In the latter case, the main division is between optical and mass spectrometry. Mass spectrometry generally gives significantly better analytical performance, but is also significantly more complex. This complexity translates into higher purchase costs, higher operational costs, more operator training, and a greater number of components that can potentially fail. Because optical spectroscopy is often less expensive and has performance adequate for many tasks, it is far more common. Atomic absorption spectrometers are one of the most commonly sold and used analytical devices.

A potential well is the region surrounding a local minimum of potential energy. Energy captured in a potential well is unable to convert to another type of energy because it is captured in the local minimum of a potential well. Therefore, a body may not proceed to the global minimum of potential energy, as it would naturally tend to do due to entropy.
Quantum-cascade lasers (QCLs) are semiconductor lasers that emit in the mid- to far-infrared portion of the electromagnetic spectrum and were first demonstrated by Jérôme Faist, Federico Capasso, Deborah Sivco, Carlo Sirtori, Albert Hutchinson, and Alfred Cho at Bell Laboratories in 1994.

Fluoride glass is a class of non-oxide optical glasses composed of fluorides of various metals. They can contain heavy metals such as zirconium, or be combined with lighter elements like aluminium and beryllium. These heavier elements cause the glass to have a transparency range extended into the infrared wavelength.

Interband cascade lasers (ICLs) are a type of laser diode that can produce coherent radiation over a large part of the mid-infrared region of the electromagnetic spectrum. They are fabricated from epitaxially-grown semiconductor heterostructures composed of layers of indium arsenide (InAs), gallium antimonide (GaSb), aluminum antimonide (AlSb), and related alloys. These lasers are similar to quantum cascade lasers (QCLs) in several ways. Like QCLs, ICLs employ the concept of bandstructure engineering to achieve an optimized laser design and reuse injected electrons to emit multiple photons. However, in ICLs, photons are generated with interband transitions, rather than the intersubband transitions used in QCLs. Consequently, the rate at which the carriers injected into the upper laser subband thermally relax to the lower subband is determined by interband Auger, radiative, and Shockley-Read carrier recombination. These processes typically occur on a much slower time scale than the longitudinal optical phonon interactions that mediates the intersubband relaxation of injected electrons in mid-IR QCLs. The use of interband transitions allows laser action in ICLs to be achieved at lower electrical input powers than is possible with QCLs.

Elias Burstein was an American experimental condensed matter physicist whose active career in science spanned seven decades. He is known for his pioneering fundamental research in the optical physics of solids; for writing and editing hundreds of articles and other publications; for bringing together scientists from around the world in international meetings, conferences, and symposia; and for training and mentoring dozens of younger physicists.
Electroreflectance is the change of reflectivity of a solid due to the influence of an electric field close to, or at the interface of the solid with a liquid. The change in reflectivity is most noticeable at very specific ranges of photon energy, corresponding to the band gaps at critical points of the Brillouin zone.













