Iron oxalate may refer to the following::
- Iron(II) oxalate, Fe(C2O4)
- Ferric oxalate, Fe2(C2O4)3
Iron oxalate may refer to the following::

Calcium oxalate (in archaic terminology, oxalate of lime) is a calcium salt of oxalic acid with the chemical formula CaC2O4 or Ca(COO)2. It forms hydrates CaC2O4·nH2O, where n varies from 1 to 3. Anhydrous and all hydrated forms are colorless or white. The monohydrate CaC2O4·H2O occurs naturally as the mineral whewellite, forming envelope-shaped crystals, known in plants as raphides. The two rarer hydrates are dihydrate CaC2O4·2H2O, which occurs naturally as the mineral weddellite, and trihydrate CaC2O4·3H2O, which occurs naturally as the mineral caoxite, are also recognized. Some foods have high quantities of calcium oxalates and can produce sores and numbing on ingestion and may even be fatal. Cultural groups with diets that depend highly on fruits and vegetables high in calcium oxalate, such as those in Micronesia, reduce the level of it by boiling and cooking them. They are a constituent in 76% of human kidney stones. Calcium oxalate is also found in beerstone, a scale that forms on containers used in breweries.

Barium oxalate is a chemical compound with the chemical formula BaC2O4. It is a barium salt of oxalic acid. It consists of barium cations Ba2+ and oxalate anions C2O2−4. It is a white odorless powder that is sometimes used as a green pyrotechnic colorant generally in specialized pyrotechnic compositions containing magnesium metal powder. Flame color is rich and vivid without additional chlorine donors. Such compositions burn rate is satisfied without commonly used oxidizers as nitrates, chlorates and perchlorates.

Potassium ferrioxalate, also called potassium trisoxalatoferrate or potassium tris(oxalato)ferrate(III) is a chemical compound with the formula K3[Fe(C2O4)3]. It often occurs as the trihydrate K3[Fe(C2O4)3]·3H2O. Both are crystalline compounds, lime green in colour.
The molecular formula C2O4 (molar mass: 88.02 g/mol) may refer to:

Ferrous oxalate (iron(II) oxalate) are inorganic compound with the formula FeC2O4(H2O)x where x is 0 or 2. These are orange compounds, poorly soluble in water.

Sodium ferrioxalate are inorganic compounds with the formula Na3Fe(C2O4)3(H2O)n. The pentahydrate has been characterized by X-ray crystallography. In contrast the potassium, ammonium, and rubidium salts crystallize from water as their trihydrates.

Cobalt(II) oxalate is the inorganic compound with the formula of CoC2O4. Like other simple inorganic oxalates, it is a coordination polymer. The oxalate ligands bridge of Co(OH2)2 centres. Each cobalt adopts octahedral coordination geometry.
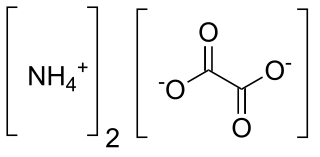
Ammonium oxalate is a chemical compound with the chemical formula [NH4]2C2O4. Its formula is often written as (NH4)2C2O4 or (COONH4)2. It is an ammonium salt of oxalic acid. It consists of ammonium cations ([NH4]+) and oxalate anions (C2O2−4). The structure of ammonium oxalate is ([NH4]+)2[C2O4]2−. Ammonium oxalate sometimes comes as a monohydrate ([NH4]2C2O4·H2O). It is a colorless or white salt under standard conditions and is odorless and non-volatile. It occurs in many plants and vegetables.

Ferric oxalate, also known as iron(III) oxalate, refers to inorganic compounds with the formula Fe2(C2O4)3(H2O)x but could also refer to salts of [Fe(C2O4)3]3-. Fe2(C2O4)3(H2O)x are coordination polymers with varying degrees of hydration. The coordination complex with the formula [Fe(C2O4)3]3- forms a variety of salts, a well-known example being potassium ferrioxalate. This article emphasizes the coordination polymers.

Strontium oxalate is a compound with the chemical formula SrC2O4. Strontium oxalate can exist either in a hydrated form or as the acidic salt of strontium oxalate.

Lead(II) oxalate is an organic compound with the formula PbC2O4. It is naturally found as a heavy white solid.

Magnesium oxalate is an organic compound comprising a magnesium cation with a 2+ charge bonded to an oxalate anion. It has the chemical formula MgC2O4. Magnesium oxalate is a white solid that comes in two forms: an anhydrous form and a dihydrate form where two water molecules are complexed with the structure. Both forms are practically insoluble in water and are insoluble in organic solutions.
Potassium ferrooxalate, also known as potassium bisoxalatoferrate(II), is a salt with the formula K2Fe(C2O4)2(H2O)x. The anion is a transition metal oxalate complex, consisting of an atom of iron in the +2 oxidation state bound to oxalate (C
2O2−
4) ligands and water.

Copper(II) oxalate are inorganic compounds with the chemical formula CuC2O4(H2O)x. The value of x can be 0, 0.44, and 1. Two of these species are found as secondary minerals, whewellite (monohydrate) and moolooite. The anhydrous compound has been characterized by X-ray crystallography. Many transition metal oxalate complexes are known.

Transition metal oxalate complexes are coordination complexes with oxalate (C2O42−) ligands. Some are useful commercially, but the topic has attracted regular scholarly scrutiny. Oxalate (C2O42-) is a kind of dicarboxylate ligand. As a small, symmetrical dinegative ion, oxalate commonly forms five-membered MO2C2 chelate rings. Mixed ligand complexes are known, e.g., [Co(C2O4)(NH3)4]κ+.
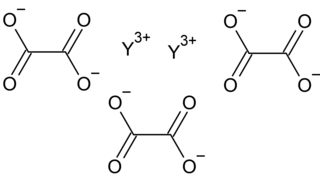
Yttrium oxalate is an inorganic compound, a salt of yttrium and oxalic acid with the chemical formula Y2(C2O4)3. The compound does not dissolve in water and forms crystalline hydrates—colorless crystals.

The oxalate phosphates are chemical compounds containing oxalate and phosphate anions. They are also called oxalatophosphates or phosphate oxalates. Some oxalate-phosphate minerals found in bat guano deposits are known. Oxalate phosphates can form metal organic framework compounds.
Manganese oxalate is a chemical compound, a salt of manganese and oxalic acid with the chemical formula MnC
2O
4. The compound creates light pink crystals, does not dissolve in water, and forms crystalline hydrates. It occurs naturally as the mineral Lindbergite.

Neodymium(III) oxalate is the oxalate salt of neodymium, with the chemical formula of Nd2(C2O4)3 in the anhydrous or hydrate form. Its decahydrate decomposes to the anhydrous form when heated, and when heated further, decomposes to Nd2O2C2O4, finally obtaining neodymium(III) oxide. It dissolves in hydrochloric acid to form Nd(C2O4)Cl·3H2O.
Europium(III) oxalate (Eu2(C2O4)3) is a chemical compound of europium and oxalic acid. There are different hydrates including the decahydrate, hexahydrate and tetrahydrate. Europium(II) oxalate is also known.