In chemistry, isovalent or second order hybridization is an extension of orbital hybridization, the mixing of atomic orbitals into hybrid orbitals which can form chemical bonds, to include fractional numbers of atomic orbitals of each type (s, p, d). It allows for a quantitative depiction of bond formation when the molecular geometry deviates from ideal bond angles.

Chemistry is the scientific discipline involved with elements and compounds composed of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances.
Only bonding with 4 equivalent substituents results in exactly sp3 hybridization. For molecules with different substituents, we can use isovalent hybridization to rationalize the differences in bond angles between different atoms. In the molecule methyl fluoride for example, the HCF bond angle (108.73°) is less than the HCH bond angle (110.2°). [1] This difference can be attributed to more p character in the C−F bonding and more s character in the C−H bonding orbitals. The hybridisation of bond orbitals is determined by Bent's rule: "Atomic s character concentrates in orbitals directed toward electropositive substituents".
In organic chemistry and biochemistry, a substituent is an atom or group of atoms which replaces one or more hydrogen atoms on the parent chain of a hydrocarbon, becoming a moiety of the resultant new molecule. The terms substituent and functional group, as well as other ones are used almost interchangeably to describe branches from a parent structure, though certain distinctions are made in the context of polymer chemistry. In polymers, side chains extend from a backbone structure. In proteins, side chains are attached to the alpha carbon atoms of the amino acid backbone.

A molecule is an electrically neutral group of two or more atoms held together by chemical bonds. Molecules are distinguished from ions by their lack of electrical charge. However, in quantum physics, organic chemistry, and biochemistry, the term molecule is often used less strictly, also being applied to polyatomic ions.
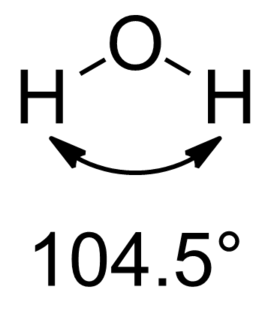
In chemistry, Bent's rule describes and explains the relationship between the orbital hybridization of central atoms in molecules and the electronegativities of substituents. The rule was stated by Henry Bent as follows:
Atomic s character concentrates in orbitals directed toward electropositive substituents.
The bond length between similar atoms also shortens with increasing s character. For example, the C−H bond length is 110.2 pm in ethane, 108.5 pm in ethylene and 106.1 pm in acetylene, with carbon hybridizations sp3 (25% s), sp2 (33% s) and sp (50% s) respectively.
Ethane is an organic chemical compound with chemical formula C
2H
6. At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is isolated on an industrial scale from natural gas and as a petrochemical by-product of petroleum refining. Its chief use is as feedstock for ethylene production.

Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula C
2H
4 or H2C=CH2. It is a colorless flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).

Acetylene (systematic name: ethyne) is the chemical compound with the formula C2H2. It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure form and thus is usually handled as a solution. Pure acetylene is odorless, but commercial grades usually have a marked odor due to impurities.
To determine the degree of hybridization of each bond one can utilize a hybridization parameter (λ). For hybrids of s and p orbitals, this is the coefficient multiplying the p orbital when the hybrid orbital is written in the form . The square of the hybridization parameter equals the hybridization index (n) of an spn orbital. [2] [3] [4] .
The fractional s character of orbital i is , and the s character of all the hybrid orbitals must sum to one, so that
The fractional p character of orbital i is , and the p character of all the hybrid orbitals sums to the number of p orbitals involved in the formation of hybrids:
These hybridization parameters can then be related to physical properties like bond angles. Using the two bonding atomic orbitals i and j we are able to find the magnitude of the interorbital angle. The orthogonality condition implies the relation known as Coulson's theorem: [5]
Charles Alfred Coulson was a British applied mathematician, theoretical chemist and religious author.
For two identical ligands the following equation can be utilized:
The hybridization index cannot be measured directly in any way. However, one can find it indirectly by measuring specific physical properties. Because nuclear spins are coupled through bonding electrons, and the electron penetration to the nucleus is dependent on s character of the hybrid orbital used in bonding, J-coupling constants determined through NMR spectroscopy is a convenient experimental parameter that can be used to estimate the hybridization index of orbitals on carbon. The relationships for one-bond 13C-1H and 13C-13C coupling are
In nuclear chemistry and nuclear physics, Scalar or J-couplings are mediated through chemical bonds connecting two spins. It is an indirect interaction between two nuclear spins which arises from hyperfine interactions between the nuclei and local electrons. In NMR spectroscopy J-coupling contains information about relative bond distances and angles. Most importantly, J-coupling provides information on the connectivity of chemical bonds. It is responsible for the often complex splitting of resonance lines in the NMR spectra of fairly simple molecules.
- and , [6]
where 1JX-Y is the one-bond NMR spin-spin coupling constant between nuclei X and Y and χS(α) is the s character of orbital α on carbon, expressed as a fraction of unity.
As an application, the 13C-1H coupling constants show that for the cycloalkanes, the amount of s character in the carbon hybrid orbital employed in the C-H bond decreases as the ring size increases. The value of 1J13C-1H for cyclopropane, cyclobutane and cyclopentane are 161, 134, and 128 Hz, respectively. This is a consequence of the fact that the C-C bonds in small, strained rings (cyclopropane and cyclobutane) employ excess p character to accommodate their molecular geometries (these bonds are famously known as 'banana bonds'). In order to conserve the total number of s and p orbitals used in hybridization for each carbon, the hybrid orbital used to form the C-H bonds must in turn compensate by taking on more s character. [2] [4] [7] Experimentally, this is also demonstrated by the significantly higher acidity of cyclopropane (pKa~ 46) compared to, for instance, cyclohexane (pKa ~ 52). [4] [8] [9]

















