
The human immunodeficiency viruses (HIV) are two species of Lentivirus that infect humans. Over time, they cause acquired immunodeficiency syndrome (AIDS), a condition in which progressive failure of the immune system allows life-threatening opportunistic infections and cancers to thrive. Without treatment, the average survival time after infection with HIV is estimated to be 9 to 11 years, depending on the HIV subtype.

A retrovirus is a type of virus that inserts a DNA copy of its RNA genome into the DNA of a host cell that it invades, thus changing the genome of that cell. After invading a host cell's cytoplasm, the virus uses its own reverse transcriptase enzyme to produce DNA from its RNA genome, the reverse of the usual pattern, thus retro (backwards). The new DNA is then incorporated into the host cell genome by an integrase enzyme, at which point the retroviral DNA is referred to as a provirus. The host cell then treats the viral DNA as part of its own genome, transcribing and translating the viral genes along with the cell's own genes, producing the proteins required to assemble new copies of the virus. Many retroviruses cause serious diseases in humans, other mammals, and birds.
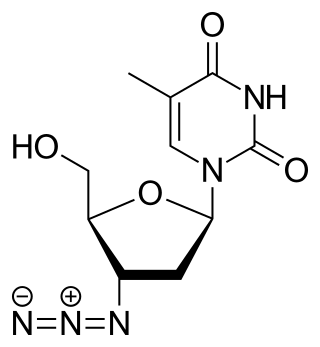
Zidovudine (ZDV), also known as azidothymidine (AZT), was the first antiretroviral medication used to prevent and treat HIV/AIDS. It is generally recommended for use in combination with other antiretrovirals. It may be used to prevent mother-to-child spread during birth or after a needlestick injury or other potential exposure. It is sold both by itself and together as lamivudine/zidovudine and abacavir/lamivudine/zidovudine. It can be used by mouth or by slow injection into a vein.
Mouse mammary tumor virus (MMTV) is a milk-transmitted retrovirus like the HTL viruses, HI viruses, and BLV. It belongs to the genus Betaretrovirus. MMTV was formerly known as Bittner virus, and previously the "milk factor", referring to the extra-chromosomal vertical transmission of murine breast cancer by adoptive nursing, demonstrated in 1936, by John Joseph Bittner while working at the Jackson Laboratory in Bar Harbor, Maine. Bittner established the theory that a cancerous agent, or "milk factor", could be transmitted by cancerous mothers to young mice from a virus in their mother's milk. The majority of mammary tumors in mice are caused by mouse mammary tumor virus.
An oncovirus or oncogenic virus is a virus that can cause cancer. This term originated from studies of acutely transforming retroviruses in the 1950–60s, when the term "oncornaviruses" was used to denote their RNA virus origin. With the letters "RNA" removed, it now refers to any virus with a DNA or RNA genome causing cancer and is synonymous with "tumor virus" or "cancer virus". The vast majority of human and animal viruses do not cause cancer, probably because of longstanding co-evolution between the virus and its host. Oncoviruses have been important not only in epidemiology, but also in investigations of cell cycle control mechanisms such as the retinoblastoma protein.

Gammaretrovirus is a genus in the Retroviridae family. Example species are the murine leukemia virus and the feline leukemia virus. They cause various sarcomas, leukemias and immune deficiencies in mammals, reptiles and birds.
Following infection with HIV-1, the rate of clinical disease progression varies between individuals. Factors such as host susceptibility, genetics and immune function, health care and co-infections as well as viral genetic variability may affect the rate of progression to the point of needing to take medication in order not to develop AIDS.
The murine leukemia viruses are retroviruses named for their ability to cause cancer in murine (mouse) hosts. Some MLVs may infect other vertebrates. MLVs include both exogenous and endogenous viruses. Replicating MLVs have a positive sense, single-stranded RNA (ssRNA) genome that replicates through a DNA intermediate via the process of reverse transcription.
Simian foamy virus (SFV) is a species of the genus Spumavirus that belongs to the family of Retroviridae. It has been identified in a wide variety of primates, including prosimians, New World and Old World monkeys, as well as apes, and each species has been shown to harbor a unique (species-specific) strain of SFV, including African green monkeys, baboons, macaques, and chimpanzees. As it is related to the more well-known retrovirus human immunodeficiency virus (HIV), its discovery in primates has led to some speculation that HIV may have been spread to the human species in Africa through contact with blood from apes, monkeys, and other primates, most likely through bushmeat-hunting practices.

Xenotropic murine leukemia virus–related virus (XMRV) is a retrovirus which was first described in 2006 as an apparently novel human pathogen found in tissue samples from men with prostate cancer. Initial reports erroneously linked the virus to prostate cancer and later to chronic fatigue syndrome (CFS), leading to considerable interest in the scientific and patient communities, investigation of XMRV as a potential cause of multiple medical conditions, and public-health concerns about the safety of the donated blood supply.

Robert Charles Gallo is an American biomedical researcher. He is best known for his role in establishing the human immunodeficiency virus (HIV) as the infectious agent responsible for acquired immune deficiency syndrome (AIDS) and in the development of the HIV blood test, and he has been a major contributor to subsequent HIV research.

Françoise Barré-Sinoussi is a French virologist and Director of the Regulation of Retroviral Infections Division and Professor at the Institut Pasteur in Paris, France. Born in Paris, France, Barré-Sinoussi performed some of the fundamental work in the identification of the human immunodeficiency virus (HIV) as the cause of AIDS. In 2008, Barré-Sinoussi was awarded the Nobel Prize in Physiology or Medicine, together with her former mentor, Luc Montagnier, for their discovery of HIV. She mandatorily retired from active research on August 31, 2015, and fully retired by some time in 2017.

The primate T-lymphotropic viruses (PTLVs) are a group of retroviruses that infect primates, using their lymphocytes to reproduce. The ones that infect humans are known as human T-lymphotropic virus (HTLV), and the ones that infect Old World monkeys are called simian T-lymphotropic viruses (STLVs). PTLVs are named for their ability to cause adult T-cell leukemia/lymphoma, but in the case of HTLV-1 it can also cause a demyelinating disease called tropical spastic paraparesis. On the other hand, newer PTLVs are simply placed into the group by similarity and their connection to human disease remains unclear.

Jonathan Paul Stoye is a virologist at the Francis Crick Institute in London, England. He has made substantial contributions to scientific understanding of the interactions of retroviruses with their hosts.
CD8+ cell noncytotoxic anti-HIV response appears to be an anti-HIV innate immune response because it can be observed in vitro with CD8+ cells from unexposed and uninfected healthy individuals.

William A. Haseltine is an American scientist, businessman, author, and philanthropist. He is known for his groundbreaking work on HIV/AIDS and the human genome.

Jacques Leibowitch was a French medical doctor and clinical researcher known for his contributions to the knowledge and treatment of HIV and AIDS, starting with his initial designation of a human retrovirus as the cause of AIDS, and his ground-breaking use of triple combination therapy for the effective control of HIV in the patient. A practicing physician in the infectiology department of the Raymond Poincaré University Hospital of Garches, University lecturer Emeritus, he led the treatment program ICCARRE that proposes a dramatic reduction of weekly anti-HIV drug intake, down to 2-3 anti-viral pills a day taken 2 to 3 or 4 days a week, as opposed to the presently recommended seven days a week, as still universally prescribed. These reduced medical dosages are adequate, necessary and sufficient according to the results of his exploratory clinical research carried out since 2003. He is the author of the books "Un virus étrange venu d'ailleurs", and "Pour en finir avec le sida".
Alexander F. Voevodin is a Russian-born biomedical scientist and educator. He is considered one of the leading early pioneers of HIV/AIDS research.
M. Juliana “Julie” McElrath is a senior vice president and director of the vaccine and infection disease division at Fred Hutchinson Cancer Research Center and the principal investigator of the HIV Vaccine Trials Network Laboratory Center in Seattle, Washington. She is also a professor at the University of Washington.
Ya-Chi Ho is a Taiwanese infectious disease researcher and Associate Professor of Microbial Pathogenesis and Medicine at Yale University. Her research centers on the interaction between HIV and the host's immune system with the ultimate goal of curing HIV/AIDS.











