
Fibronectin is a high-molecular weight glycoprotein of the extracellular matrix that binds to membrane-spanning receptor proteins called integrins. Fibronectin also binds to other extracellular matrix proteins such as collagen, fibrin, and heparan sulfate proteoglycans.

In biology, the extracellular matrix (ECM) is a three-dimensional network consisting of extracellular macromolecules and minerals, such as collagen, enzymes, glycoproteins and hydroxyapatite that provide structural and biochemical support to surrounding cells. Because multicellularity evolved independently in different multicellular lineages, the composition of ECM varies between multicellular structures; however, cell adhesion, cell-to-cell communication and differentiation are common functions of the ECM.
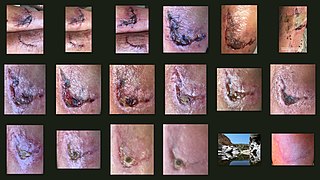
Wound healing refers to a living organism's replacement of destroyed or damaged tissue by newly produced tissue.

Cell adhesion is the process by which cells interact and attach to neighbouring cells through specialised molecules of the cell surface. This process can occur either through direct contact between cell surfaces such as cell junctions or indirect interaction, where cells attach to surrounding extracellular matrix, a gel-like structure containing molecules released by cells into spaces between them. Cells adhesion occurs from the interactions between cell-adhesion molecules (CAMs), transmembrane proteins located on the cell surface. Cell adhesion links cells in different ways and can be involved in signal transduction for cells to detect and respond to changes in the surroundings. Other cellular processes regulated by cell adhesion include cell migration and tissue development in multicellular organisms. Alterations in cell adhesion can disrupt important cellular processes and lead to a variety of diseases, including cancer and arthritis. Cell adhesion is also essential for infectious organisms, such as bacteria or viruses, to cause diseases.
Cell adhesion molecules (CAMs) are a subset of cell surface proteins that are involved in the binding of cells with other cells or with the extracellular matrix (ECM), in a process called cell adhesion. In essence, CAMs help cells stick to each other and to their surroundings. CAMs are crucial components in maintaining tissue structure and function. In fully developed animals, these molecules play an integral role in generating force and movement and consequently ensuring that organs are able to execute their functions normally. In addition to serving as "molecular glue", CAMs play important roles in the cellular mechanisms of growth, contact inhibition, and apoptosis. Aberrant expression of CAMs may result in a wide range of pathologies, ranging from frostbite to cancer.
Biological crosstalk refers to instances in which one or more components of one signal transduction pathway affects another. This can be achieved through a number of ways with the most common form being crosstalk between proteins of signaling cascades. In these signal transduction pathways, there are often shared components that can interact with either pathway. A more complex instance of crosstalk can be observed with transmembrane crosstalk between the extracellular matrix (ECM) and the cytoskeleton.

Tenascins are extracellular matrix glycoproteins. They are abundant in the extracellular matrix of developing vertebrate embryos and they reappear around healing wounds and in the stroma of some tumors.

Integrin alpha-3 is a protein that in humans is encoded by the ITGA3 gene. ITGA3 is an integrin alpha subunit. Together with beta-1 subunit, it makes up half of the α3β1 integrin duplex that plays a role in neural migration and corticogenesis, acted upon by such factors as netrin-1 and reelin.

Fibulin (FY-beau-lin) is the prototypic member of a multigene family, currently with seven members. Fibulin-1 is a calcium-binding glycoprotein. In vertebrates, fibulin-1 is found in blood and extracellular matrices. In the extracellular matrix, fibulin-1 associates with basement membranes and elastic fibers. The association with these matrix structures is mediated by its ability to interact with numerous extracellular matrix constituents including fibronectin, proteoglycans, laminins and tropoelastin. In blood, fibulin-1 binds to fibrinogen and incorporates into clots.

FBLN1 is the gene encoding fibulin-1, an extracellular matrix and plasma protein.

Integrin alpha-5 is a protein that in humans is encoded by the ITGA5 gene.

Tenascin C (TN-C) is a glycoprotein that in humans is encoded by the TNC gene. It is expressed in the extracellular matrix of various tissues during development, disease or injury, and in restricted neurogenic areas of the central nervous system. Tenascin-C is the founding member of the tenascin protein family. In the embryo it is made by migrating cells like the neural crest; it is also abundant in developing tendons, bone and cartilage.

Collagen alpha-4(IV) chain is a protein that in humans is encoded by the COL4A4 gene.

Collagen alpha-2(IV) chain is a protein that in humans is encoded by the COL4A2 gene.

Laminin subunit alpha-1 is a protein that in humans is encoded by the LAMA1 gene.

Collagen alpha-6(IV) chain is a protein that in humans is encoded by the COL4A6 gene.

Fibulin-2 is a protein that in humans is encoded by the FBLN2 gene.
Collagen VI (ColVI) is a type of collagen primarily associated with the extracellular matrix of skeletal muscle. ColVI maintains regularity in muscle function and stabilizes the cell membrane. It is synthesized by a complex, multistep pathway that leads to the formation of a unique network of linked microfilaments located in the extracellular matrix (ECM). ColVI plays a vital role in numerous cell types, including chondrocytes, neurons, myocytes, fibroblasts, and cardiomyocytes. ColVI molecules are made up of three alpha chains: α1(VI), α2(VI), and α3(VI). It is encoded by 6 genes: COL6A1, COL6A2, COL6A3, COL6A4, COL6A5, and COL6A6. The chain lengths of α1(VI) and α2(VI) are about 1,000 amino acids. The chain length of α3(VI) is roughly a third larger than those of α1(VI) and α2(VI), and it consists of several spliced variants within the range of 2,500 to 3,100 amino acids.
A. Hari Reddi is a Distinguished Professor and holder of the Lawrence J. Ellison Endowed Chair in Musculoskeletal Molecular Biology at the University of California, Davis. He was previously the Virginia M. and William A. Percy Chair and Professor in Orthopaedic Surgery, Professor of Biological Chemistry, and Professor of Oncology at the Johns Hopkins University School of Medicine. Professor Reddi's research played an indispensable role in the identification, isolation and purification of bone morphogenetic proteins (BMPs) that are involved in bone formation and repair. The molecular mechanism of bone induction studied by Professor Reddi led to the conceptual advance in tissue engineering that morphogens/metabologens bound to an insoluble extracellular matrix scaffolding act in collaboration to stimulate stem cells to form cartilage and bone. The Reddi laboratory has also made important discoveries unraveling the role of the extracellular matrix in bone and cartilage tissue regeneration and repair.
Ruth Chiquet-Ehrismann was a Swiss biochemist and cell biologist working on interactions in the extracellular matrix.















