
Bradykinin (BK) is a peptide that promotes inflammation. It causes arterioles to dilate (enlarge) via the release of prostacyclin, nitric oxide, and endothelium-derived hyperpolarizing factor and makes veins constrict, via prostaglandin F2, thereby leading to leakage into capillary beds, due to the increased pressure in the capillaries. Bradykinin is a physiologically and pharmacologically active peptide of the kinin group of proteins, consisting of nine amino acids.
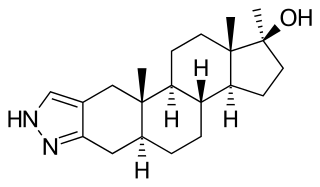
Stanozolol, sold under many brand names, is an androgen and anabolic steroid (AAS) medication derived from dihydrotestosterone (DHT). It is used to treat hereditary angioedema. It was developed by American pharmaceutical company Winthrop Laboratories in 1962, and has been approved by the U.S. Food and Drug Administration for human use, though it is no longer marketed in the USA. It is also used in veterinary medicine. Stanozolol has mostly been discontinued, and remains available in only a few countries. It is given by mouth in humans or by injection into muscle in animals.
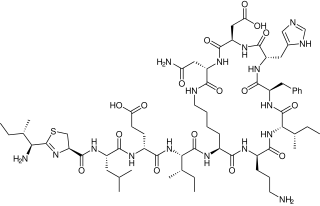
Bacitracin is a polypeptide antibiotic. It is a mixture of related cyclic peptides produced by Bacillus licheniformis bacteria, that was first isolated from the variety "Tracy I" in 1945. These peptides disrupt Gram-positive bacteria by interfering with cell wall and peptidoglycan synthesis.
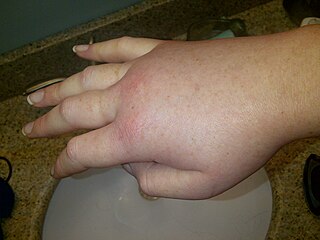
Hereditary angioedema (HAE) is a disorder that results in recurrent attacks of severe swelling. The swelling most commonly affects the arms, legs, face, intestinal tract, and airway. If the intestinal tract is affected, abdominal pain and vomiting may occur. Swelling of the airway can result in its obstruction and trouble breathing. Without preventive treatment, attacks typically occur every two weeks and last for a few days.
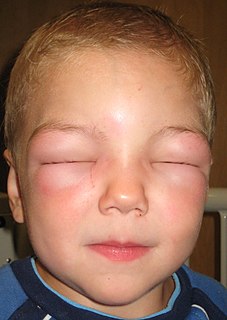
Angioedema is an area of swelling (edema) of the lower layer of skin and tissue just under the skin or mucous membranes. The swelling may occur in the face, tongue, larynx, abdomen, or arms and legs. Often it is associated with hives, which are swelling within the upper skin. Onset is typically over minutes to hours.
Antisense therapy is a form of treatment that uses antisense oligonucleotides (ASOs) to target messenger RNA (mRNA). ASOs are capable of altering mRNA expression through a variety of mechanisms, including ribonuclease H mediated decay of the pre-mRNA, direct steric blockage, and exon content modulation through splicing site binding on pre-mRNA. Several ASOs have been approved in the United States, the European Union, and elsewhere.
Astex Pharmaceuticals ("Astex") is a biotechnology company focused on the discovery and development of drugs in oncology and diseases of the central nervous system. Astex was founded in 1999 by Sir Tom Blundell, Chris Abell & Harren Jhoti, and is located in Cambridge, England.

ViroPharma Incorporated was a pharmaceutical company that developed and sold drugs that addressed serious diseases treated by physician specialists and in hospital settings. The company focused on product development activities on viruses and human disease, including those caused by cytomegalovirus (CMV) and hepatitis C virus (HCV) infections. It was purchased by Shire in 2013, with Shire paying around $4.2 billion for the company in a deal that was finalized in January 2014. ViroPharma was a member of the NASDAQ Biotechnology Index and the S&P 600.
A protein kinase inhibitor is a type of enzyme inhibitor that blocks the action of one or more protein kinases. Protein kinases are enzymes that phosphorylate (add a phosphate, or PO4) group to a protein, and can modulate its function.
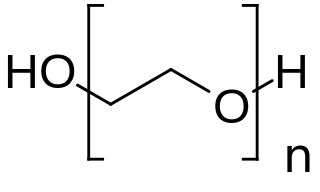
PEGylation is the process of both covalent and non-covalent attachment or amalgamation of polyethylene glycol polymer chains to molecules and macrostructures, such as a drug, therapeutic protein or vesicle, which is then described as PEGylated. PEGylation affects the resulting derivatives or aggregates interactions, which typically slows down their coalescence and degradation as well as elimination in vivo.

Icatibant, sold under the brand name Firazyr, is a medication for the symptomatic treatment of acute attacks of hereditary angioedema (HAE) in adults with C1-esterase-inhibitor deficiency. It is not effective in angioedema caused by medication from the ACE inhibitor class.

Liraglutide, sold under the brand name Victoza among others, is an anti-diabetic medication used to treat type 2 diabetes, obesity, and chronic weight management. In diabetes it is a less preferred agent compared to metformin. Its effects on long-term health outcomes like heart disease and life expectancy are unclear. It is given by injection under the skin.
Ecallantide is a drug used for the treatment of hereditary angioedema (HAE) and in the prevention of blood loss in cardiothoracic surgery. It is an inhibitor of the protein kallikrein and a 60-amino acid polypeptide which was developed from a Kunitz domain through phage display to mimic antibodies inhibiting kallikrein.
BioCryst Pharmaceuticals, Inc. is an American pharmaceutical company headquartered in Durham, North Carolina. The company is a late stage biotech company that focuses on oral drugs for rare and serious diseases. BioCryst's antiviral drug peramivir (Rapivab) was approved by FDA in December 2014. It has also been approved in Japan, Korea, and China.

Tecovirimat, sold under the brand name Tpoxx among others, is an antiviral medication with activity against orthopoxviruses such as smallpox and monkeypox. It is the first antipoxviral drug approved in the United States. It is an inhibitor of the orthopoxvirus VP37 envelope wrapping protein.

Antibody-drug conjugates or ADCs are a class of biopharmaceutical drugs designed as a targeted therapy for treating cancer. Unlike chemotherapy, ADCs are intended to target and kill tumor cells while sparing healthy cells. As of 2019, some 56 pharmaceutical companies were developing ADCs.
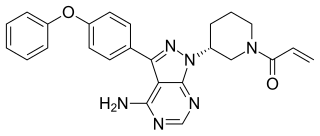
Ibrutinib, sold under the brand name Imbruvica among others, is a small molecule drug that inhibits B-cell proliferation and survival by irreversibly binding the protein Bruton's tyrosine kinase (BTK). Blocking BTK inhibits the B-cell receptor pathway, which is often aberrantly active in B cell cancers. Ibrutinib is therefore used to treat such cancers, including mantle cell lymphoma, chronic lymphocytic leukemia, and Waldenström's macroglobulinemia.
Brilacidin, an investigational new drug (IND), is a polymer-based antibiotic currently in human clinical trials, and represents a new class of antibiotics called host defense protein mimetics, or HDP-mimetics, which are non-peptide synthetic small molecules modeled after host defense peptides (HDPs). HDPs, also called antimicrobial peptides, some of which are defensins, are part of the innate immune response and are common to most higher forms of life. As brilacidin is modeled after a defensin, it is also called a defensin mimetic.
Lanadelumab is a human monoclonal antibody that targets plasma kallikrein (pKal) in order to promote prevention of angioedema in patients with hereditary angioedema. Lanadelumab, was approved in the United States as the first monoclonal antibody indicated for prophylactic treatment to prevent hereditary angioedema (HAE) attacks. Takhzyro is the first treatment for hereditary angioedema (HEA) prevention made by using cells within a lab, not human plasma. The US Food and Drug Administration approved the use of lanadelumab on 23 August 2018 for patients that are 12 years and older and have either type I or type II hereditary angioedema (HEA). Administration of the medication is done through 1 subcutaneous injection at a dose of 300 milligrams every 2 weeks.

Berotralstat, sold under the brand name Orladeyo, is a medication used to prevent attacks of hereditary angioedema (HAE) in people aged twelve years and older.













