Related Research Articles

Rubber, also called India rubber, latex, Amazonian rubber, caucho, or caoutchouc, as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds. Thailand, Malaysia, and Indonesia are three of the leading rubber producers.
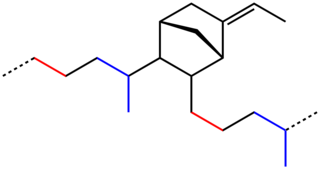
EPDM rubber is a type of synthetic rubber that is used in many applications. Dienes used in the manufacture of EPDM rubbers are ethylidene norbornene (ENB), dicyclopentadiene (DCPD), and vinyl norbornene (VNB). 4-8% of these monomers are typically used.

The School of Engineering is one of the ten schools that comprise Tufts University. The school offers undergraduate and graduate degrees in several engineering disciplines and computer science fields. Along with the School of Arts and Sciences (A&S) and the Fletcher School of Law and Diplomacy, the School of Engineering is located on the university's main campus in Medford and Somerville, Massachusetts. Currently, the engineering school enrolls more than 800 full-time undergraduates and 600 graduate students. The school employs over 100 full-time and part-time faculty members.
Krishna Kumar is an Indian American chemist whose research spans organic chemistry, chemical biology, bioorganic chemistry, biophysics and cell biology. He is currently Robinson Professor of Chemistry and was also Chemistry Department Chair from 2006 to 2009; and from 2012 to 2018 at Tufts University.

The Charles Goodyear Medal is the highest honor conferred by the American Chemical Society, Rubber Division. Established in 1941, the award is named after Charles Goodyear, the discoverer of vulcanization, and consists of a gold medal, a framed certificate and prize money. The medal honors individuals for "outstanding invention, innovation, or development which has resulted in a significant change or contribution to the nature of the rubber industry". Awardees give a lecture at an ACS Rubber Division meeting, and publish a review of their work in the society's scientific journal Rubber Chemistry and Technology.

Rubber Chemistry and Technology is a quarterly peer-reviewed scientific journal covering fundamental research and technical developments relating to chemistry, materials science, and engineering of rubber, elastomers, and related materials. It was established in 1928, with Carroll C. Davis as its first editor-in-chief. The current editor-in-chief is Christopher G. Robertson. The journal is published by the American Chemical Society's Rubber Division. The journal currently publishes four issues per year containing original research contributions and review articles.
Adolf Schallamach (1905–1997) was a scientist at the British Rubber Producers' Research Association noted for pioneering understanding of the mechanisms of rubber friction. He was one of only two electrical engineers ever to win the Charles Goodyear Medal.

Stuart Blank Levy was a researcher and physician at Tufts University. He was among the first to advocate for greater awareness of antibiotic resistance and founded the Alliance for the Prudent Use of Antibiotics.
Alan D. Roberts is a Tun Abdul Razak Research Centre (TARRC) scientist noted for his contributions to understanding contact phenomena in elastomers, and in particular the JKR equation.
David Mahan Knipe is the Higgins Professor of Microbiology and Molecular Genetics in the Department of Microbiology at the Harvard Medical School in Boston, Massachusetts and co-chief editor of the reference book Fields Virology. He returned to the Chair of the Program in Virology at Harvard Medical School in 2019, having previously held the position from 2004 through 2016 and served as interim Co-Chair of the Microbiology and Immunobiology Department from 2016 through 2018.

Kuzhikalail M. Abraham is an American scientist, a recognized expert on lithium-ion and lithium-ion polymer batteries and is the inventor of the ultrahigh energy density lithium–air battery. Abraham is the principal of E-KEM Sciences in Needham, Massachusetts and a professor at the Northeastern University Center for Renewable Energy Technologies, Northeastern University, in Boston, Massachusetts.

Vinyltriethoxysilane is an organosilicon compound with the formula (C2H5O)3SiCH=CH2. It is a colorless liquid. The compound is bifunctional, featuring both a vinyl group and hydrolytically sensitive ethoxysilyl groups. As such it is a crosslinking agent.
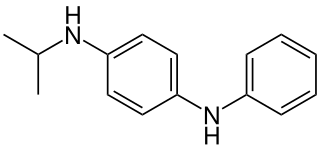
N-Isopropyl-N′-phenyl-1,4-phenylenediamine (often abbreviated IPPD) is an organic compound commonly used as an antiozonant in rubbers, particularly those used for tires. Like other p-phenylenediamine-based antiozonants it works by virtue of its low ionization energy, which allows it to react with ozone faster than ozone will react with rubber. This reaction converts it to the corresponding aminoxyl radical (R2N–O•), with the ozone being converted to a hydroperoxyl radical (HOO•), these species can then be scavenged by other antioxidant polymer stabilizers.
Ellen Marie Arruda is an American mechanical engineer known for her research on the mechanical properties of polymers and on tissue engineering, with applications including the design of improved football helmets, artificial tooth enamel that can withstand high-shock and high-vibration environments, and nanolayered composite materials that are lightweight, as strong as steel, and transparent. The Arruda–Boyce model for the behavior of rubber-like polymers is named for her and her doctoral advisor Mary Cunningham Boyce, with whom she published it in 1993. She is Maria Comninou Collegiate Professor of Mechanical Engineering and Tim Manganello / Borg Warner Department Chair of Mechanical Engineering at the University of Michigan.

6PPD is an organic chemical widely used as stabilising additive in rubbers, such as NR, SBR and BR; all of which are common in vehicle tires. Although it is an effective antioxidant it is primarily used because of its excellent antiozonant peformance. It is one of several antiozonants based around p-phenylenediamine (PPD).

ChoKyun Rha was a Korean-born American food technologist, inventor, and professor of biomaterials science and engineering at the Massachusetts Institute of Technology (MIT). She was the first Asian woman awarded tenure at MIT.
Eli Mercer Dannenberg was a Cabot scientist known for contributions to surface chemistry of carbon black
Avrom Izak Medalia was a Cabot scientist known for contributions to understanding electrical conductivity and dynamic properties of carbon black filled rubbers
Alan Hugh Muhr is a retired TARRC scientist noted for contributions to understanding the mechanics elastomer applications, including laminated rubber isolators, marine fenders, automotive mounts, and structural energy dissipation systems
Henry L. Hsieh was a Phillips Petroleum scientist known for contributions to polymerization chemistry, specifically anionic polymerization
References
- ↑ "History of Simplex".
- ↑ "History of Simplex Wire and Cable". Answers.com .
- ↑ Boggs, C. R; Blake, J. T. (1937). "Deproteinized Rubber". Rubber Chemistry and Technology. 10 (2): 288–298. doi:10.5254/1.3538979.
- ↑ Blake, J. T.; Boggs, C. R. (1926). "The Absorption of Water by Rubber". Industrial & Engineering Chemistry. 18 (3): 224–232. doi:10.1021/ie50195a002.
- ↑ Blake, J. T. (1949). "The Effects of Light and Ozone on Rubber". In Symposium on Aging of Rubbers, Am. Soc. Testing Mats: 48–48–11. doi:10.1520/STP43956S. ISBN 978-0-8031-6063-7.
- ↑ Blake, J. T.; Kitchin, D. W.; Pratt, O. S. (1955). "The Microbiological Deterioration of Rubber Insulation". Applied Microbiology. 3 (1): 35–39. doi:10.1128/am.3.1.35-39.1955. PMC 1057050 . PMID 16349572.
- ↑ Blake, J. T.; Kitchin D. W.; Pratt, O. S (1951). "Failures of Rubber Insulation Caused by Soil Microörganisms". Rubber Chemistry and Technology. 24 (2): 399–413. doi:10.5254/1.3543071. S2CID 51671879.
- ↑ "Interview with John Blake".
- ↑ "Blake Obituary". Archived from the original on 2013-10-12.