Related Research Articles
Carbonic acid is a chemical compound with the chemical formula H2CO3. The molecule rapidly converts to water and carbon dioxide in the presence of water. However, in the absence of water, it is quite stable at room temperature. The interconversion of carbon dioxide and carbonic acid is related to the breathing cycle of animals and the acidification of natural waters.
In chemistry, an acid dissociation constant is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction

In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
Solubility equilibrium is a type of dynamic equilibrium that exists when a chemical compound in the solid state is in chemical equilibrium with a solution of that compound. The solid may dissolve unchanged, with dissociation, or with chemical reaction with another constituent of the solution, such as acid or alkali. Each solubility equilibrium is characterized by a temperature-dependent solubility product which functions like an equilibrium constant. Solubility equilibria are important in pharmaceutical, environmental and many other scenarios.
Extractive metallurgy is a branch of metallurgical engineering wherein process and methods of extraction of metals from their natural mineral deposits are studied. The field is a materials science, covering all aspects of the types of ore, washing, concentration, separation, chemical processes and extraction of pure metal and their alloying to suit various applications, sometimes for direct use as a finished product, but more often in a form that requires further working to achieve the given properties to suit the applications.
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate ligand and a single central metal atom. These ligands are called chelants, chelators, chelating agents, or sequestering agents. They are usually organic compounds, but this is not a necessity.

Copper(II) nitrate describes any member of the family of inorganic compounds with the formula Cu(NO3)2(H2O)x. The hydrates are blue solids. Anhydrous copper nitrate forms blue-green crystals and sublimes in a vacuum at 150-200 °C. Common hydrates are the hemipentahydrate and trihydrate.
The equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium, a state approached by a dynamic chemical system after sufficient time has elapsed at which its composition has no measurable tendency towards further change. For a given set of reaction conditions, the equilibrium constant is independent of the initial analytical concentrations of the reactant and product species in the mixture. Thus, given the initial composition of a system, known equilibrium constant values can be used to determine the composition of the system at equilibrium. However, reaction parameters like temperature, solvent, and ionic strength may all influence the value of the equilibrium constant.
In thermodynamics, an activity coefficient is a factor used to account for deviation of a mixture of chemical substances from ideal behaviour. In an ideal mixture, the microscopic interactions between each pair of chemical species are the same and, as a result, properties of the mixtures can be expressed directly in terms of simple concentrations or partial pressures of the substances present e.g. Raoult's law. Deviations from ideality are accommodated by modifying the concentration by an activity coefficient. Analogously, expressions involving gases can be adjusted for non-ideality by scaling partial pressures by a fugacity coefficient.

Copper monosulfide is a chemical compound of copper and sulfur. It was initially thought to occur in nature as the dark indigo blue mineral covellite. However, it was later shown to be rather a cuprous compound, formula Cu+3S(S2). CuS is a moderate conductor of electricity. A black colloidal precipitate of CuS is formed when hydrogen sulfide, H2S, is bubbled through solutions of Cu(II) salts. It is one of a number of binary compounds of copper and sulfur (see copper sulfide for an overview of this subject), and has attracted interest because of its potential uses in catalysis and photovoltaics.

The uranyl ion is an oxycation of uranium in the oxidation state +6, with the chemical formula UO2+
2. It has a linear structure with short U–O bonds, indicative of the presence of multiple bonds between uranium and oxygen. Four or more ligands may be bound to the uranyl ion in an equatorial plane around the uranium atom. The uranyl ion forms many complexes, particularly with ligands that have oxygen donor atoms. Complexes of the uranyl ion are important in the extraction of uranium from its ores and in nuclear fuel reprocessing.

Copper(II) bromide (CuBr2) is a chemical compound that forms an unstable tetrahydrate CuBr2·4H2O. It is used in photographic processing as an intensifier and as a brominating agent in organic synthesis.
The word hydrolysis is applied to chemical reactions in which a substance reacts with water. In organic chemistry, the products of the reaction are usually molecular, being formed by combination with H and OH groups. In inorganic chemistry, the word most often applies to cations forming soluble hydroxide or oxide complexes with, in some cases, the formation of hydroxide and oxide precipitates.
In coordination chemistry, a stability constant is an equilibrium constant for the formation of a complex in solution. It is a measure of the strength of the interaction between the reagents that come together to form the complex. There are two main kinds of complex: compounds formed by the interaction of a metal ion with a ligand and supramolecular complexes, such as host–guest complexes and complexes of anions. The stability constant(s) provide(s) the information required to calculate the concentration(s) of the complex(es) in solution. There are many areas of application in chemistry, biology and medicine.
The Geochemist's Workbench (GWB) is an integrated set of interactive software tools for solving a range of problems in aqueous chemistry. The graphical user interface simplifies the use of the geochemical code.
Geochemical modeling or theoretical geochemistry is the practice of using chemical thermodynamics, chemical kinetics, or both, to analyze the chemical reactions that affect geologic systems, commonly with the aid of a computer. It is used in high-temperature geochemistry to simulate reactions occurring deep in the Earth's interior, in magma, for instance, or to model low-temperature reactions in aqueous solutions near the Earth's surface, the subject of this article.
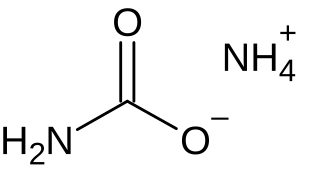
Ammonium carbamate is a chemical compound with the formula [NH4][H2NCO2] consisting of ammonium cation NH+4 and carbamate anion NH2COO−. It is a white solid that is extremely soluble in water, less so in alcohol. Ammonium carbamate can be formed by the reaction of ammonia NH3 with carbon dioxide CO2, and will slowly decompose to those gases at ordinary temperatures and pressures. It is an intermediate in the industrial synthesis of urea (NH2)2CO, an important fertilizer.
Aqion is a hydrochemistry software tool. It bridges the gap between scientific software and the calculation/handling of "simple" water-related tasks in daily routine practice. The software aqion is free for private users, education and companies.

Protodeboronation, or protodeborylation is a chemical reaction involving the protonolysis of a boronic acid in which a carbon-boron bond is broken and replaced with a carbon-hydrogen bond. Protodeboronation is a well-known undesired side reaction, and frequently associated with metal-catalysed coupling reactions that utilise boronic acids. For a given boronic acid, the propensity to undergo protodeboronation is highly variable and dependent on various factors, such as the reaction conditions employed and the organic substituent of the boronic acid.
Mineral processing and extraction of metals are very energy-intensive processes, which are not exempted of producing large volumes of solid residues and wastewater, which also require energy to be further treated and disposed. Moreover, as the demand for metals increases, the metallurgical industry must rely on sources of materials with lower metal contents both from a primary and/or secondary raw materials. Consequently, mining activities and waste recycling must evolve towards the development of more selective, efficient and environmentally friendly mineral and metal processing routes.
References
- ↑ May, PM; Murray, K (1991). "JESS, A joint expert speciation system-I. Raison d'être". Talanta. 38 (12): 1409–1417. doi:10.1016/0039-9140(91)80289-c. PMID 18965317.
- 1 2 May, PM; Rowland, D (2017). "JESS, a Joint Expert Speciation System – VI: thermodynamically-consistent standard Gibbs energies of reaction for aqueous solutions". New J. Chem. 42 (10): 7617–7629. doi:10.1039/c7nj03597g. S2CID 102538032.
- ↑ May, PM; Rowland, D (2017). "Thermodynamic Modeling of Aqueous Electrolyte Systems: Current Status". J. Chem. Eng. Data. 62 (9): 2481–2495. doi:10.1021/acs.jced.6b01055.
- ↑ Hill, MG; Königsberger, E; May, PM (2017). "Mineral precipitation and dissolution in the kidney". Am. Mineral. 102 (4): 701–710. Bibcode:2017AmMin.102..701H. doi:10.2138/am-2017-5778.
- ↑ Königsberger, L-C; et al. (2015). "Formation constants of copper(I) complexes with cysteine, penicillamine and glutathione: implications for copper speciation in the human eye". Dalton Trans. 44 (47): 20413–20425. doi:10.1039/c5dt02129d.
- ↑ Bonet, J; Filella, M; May, PM; May, RF; Murray, K (2023). "JESS Thermodynamic database of chemical reactions v8.9". Zenodo. doi:10.5281/zenodo.7700023.