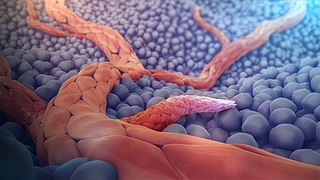
Angiogenesis is the physiological process through which new blood vessels form from pre-existing vessels, formed in the earlier stage of vasculogenesis. Angiogenesis continues the growth of the vasculature mainly by processes of sprouting and splitting, but processes such as coalescent angiogenesis, vessel elongation and vessel cooption also play a role. Vasculogenesis is the embryonic formation of endothelial cells from mesoderm cell precursors, and from neovascularization, although discussions are not always precise. The first vessels in the developing embryo form through vasculogenesis, after which angiogenesis is responsible for most, if not all, blood vessel growth during development and in disease.

The lymphatic system, or lymphoid system, is an organ system in vertebrates that is part of the immune system, and complementary to the circulatory system. It consists of a large network of lymphatic vessels, lymph nodes, lymphoid organs, lymphoid tissues and lymph. Lymph is a clear fluid carried by the lymphatic vessels back to the heart for re-circulation.

Metastasis is a pathogenic agent's spread from an initial or primary site to a different or secondary site within the host's body; the term is typically used when referring to metastasis by a cancerous tumor. The newly pathological sites, then, are metastases (mets). It is generally distinguished from cancer invasion, which is the direct extension and penetration by cancer cells into neighboring tissues.

The endothelium is a single layer of squamous endothelial cells that line the interior surface of blood vessels and lymphatic vessels. The endothelium forms an interface between circulating blood or lymph in the lumen and the rest of the vessel wall. Endothelial cells form the barrier between vessels and tissue and control the flow of substances and fluid into and out of a tissue.
Endothelial progenitor cell is a term that has been applied to multiple different cell types that play roles in the regeneration of the endothelial lining of blood vessels. Outgrowth endothelial cells are an EPC subtype committed to endothelial cell formation. Despite the history and controversy, the EPC in all its forms remains a promising target of regenerative medicine research.
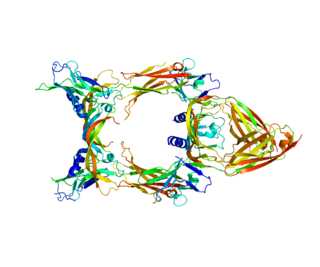
Vascular endothelial growth factor C (VEGF-C) is a protein that is a member of the platelet-derived growth factor / vascular endothelial growth factor (PDGF/VEGF) family. It is encoded in humans by the VEGFC gene, which is located on chromosome 4q34.

Kinesin-like protein KIF3B is a protein that in humans is encoded by the KIF3B gene. KIF3B is an N-type protein that complexes with two other kinesin proteins to form two-headed anterograde motors. First, KIF3B forms a heterodimer with KIF3A ; (KIF3A/3B), that is membrane-bound and has ATPase activity. Then KIFAP3 binds to the tail domain to form a heterotrimeric motor. This motor has a plus end-directed microtubule sliding activity that exhibits a velocity of ∼0.3 μm/s a. There are 14 kinesin protein families in the kinesin superfamily and KIF3B is part of the Kinesin-2 family, of kinesins that can all form heterotrimeric complexes. Expression of the three motor subunits is ubiquitous. The KIG3A/3B/KAP3 motors can transport 90 to 160 nm in diameter organelles.

EGF-like domain-containing protein 7 is a protein that in humans is encoded by the EGFL7 gene. Intron 7 of EGFL7 hosts the miR-126 microRNA gene.
Tumor-associated macrophages (TAMs) are a class of immune cells present in high numbers in the microenvironment of solid tumors. They are heavily involved in cancer-related inflammation. Macrophages are known to originate from bone marrow-derived blood monocytes or yolk sac progenitors, but the exact origin of TAMs in human tumors remains to be elucidated. The composition of monocyte-derived macrophages and tissue-resident macrophages in the tumor microenvironment depends on the tumor type, stage, size, and location, thus it has been proposed that TAM identity and heterogeneity is the outcome of interactions between tumor-derived, tissue-specific, and developmental signals.
Extracellular RNA (exRNA) describes RNA species present outside of the cells in which they were transcribed. Carried within extracellular vesicles, lipoproteins, and protein complexes, exRNAs are protected from ubiquitous RNA-degrading enzymes. exRNAs may be found in the environment or, in multicellular organisms, within the tissues or biological fluids such as venous blood, saliva, breast milk, urine, semen, menstrual blood, and vaginal fluid. Although their biological function is not fully understood, exRNAs have been proposed to play a role in a variety of biological processes including syntrophy, intercellular communication, and cell regulation. The United States National Institutes of Health (NIH) published in 2012 a set of Requests for Applications (RFAs) for investigating extracellular RNA biology. Funded by the NIH Common Fund, the resulting program was collectively known as the Extracellular RNA Communication Consortium (ERCC). The ERCC was renewed for a second phase in 2019.

Tumor-associated endothelial cells or tumor endothelial cells (TECs) refers to cells lining the tumor-associated blood vessels that control the passage of nutrients into surrounding tumor tissue. Across different cancer types, tumor-associated blood vessels have been discovered to differ significantly from normal blood vessels in morphology, gene expression, and functionality in ways that promote cancer progression. There has been notable interest in developing cancer therapeutics that capitalize on these abnormalities of the tumor-associated endothelium to destroy tumors.
A cancer-associated fibroblast (CAF) is a cell type within the tumor microenvironment that promotes tumorigenic features by initiating the remodelling of the extracellular matrix or by secreting cytokines. CAFs are a complex and abundant cell type within the tumour microenvironment; the number cannot decrease, as they are unable to undergo apoptosis.

Kairbaan Hodivala-Dilke, FMedSci is an English cell biologist who has made significant contributions to the understanding of the cellular and molecular biology of the tumour microenvironment and in particular angiogenesis. She is Professor of Angiogenesis and the Tumour Microenvironment and Deputy Institute Director of Barts Cancer Institute, Queen Mary University of London. In 2015 she was awarded the Hooke medal from the British Society for Cell Biology and EMBO membership.
Renata Homem de Gouveia Xavier de Basto is a researcher in cell and developmental biology. She is currently a team leader at the Institut Curie in Paris. She is also the deputy director of the CNRS research Unit UMR144 'Cell biology and cancer' at the Institut Curie which, comprises 14 research teams.
Axel Behrens is a German-British molecular biologist and an expert in cancer stem cell biology. He is the Scientific Director of the Cancer Research UK Convergence Science Centre in London, a senior group leader at the Institute of Cancer Research and a professor at Imperial College London.
Christiana Ruhrberg is a German-British cell biologist who is Professor of Neuronal and Vascular Biology, University College London. She looks to understand how cells interact during the development and disease of mammals.
Serena Nik-Zainal is a British-Malaysian clinician who is a consultant in clinical genetics and Cancer Research UK advanced clinician scientist at the University of Cambridge. She makes use of genomics for clinical applications. She was awarded the Crick Lecture by the Royal Society in 2021.
Judith Klein-Seetharaman is an American-German biochemist who is a professor at the Arizona State University. Her research considers the structure-function properties of proteins using computational bio-linguistics. She was supported by the Bill & Melinda Gates Foundation to identify novel therapies to tackle HIV.
Lisa M. Coussens is an American cancer scientist who is Chair of the Department of Cell, Developmental and Cancer Biology and Professor and Associate Director for Basic Research in the Knight Cancer Institute at the Oregon Health & Science University. She serves as President of the American Association for Cancer Research.
Anjali Kusumbe is a British-Indian biologist who is the Head of the Tissue and Tumour Microenvironments Group at the Medical Research Council Human Immunology Unit and Weatherall Institute of Molecular Medicine at the University of Oxford. She was awarded the Royal Microscopical Society Award for Life Sciences in 2022.








