
A fluorescence microscope is an optical microscope that uses fluorescence instead of, or in addition to, scattering, reflection, and attenuation or absorption, to study the properties of organic or inorganic substances. "Fluorescence microscope" refers to any microscope that uses fluorescence to generate an image, whether it is a simple set up like an epifluorescence microscope or a more complicated design such as a confocal microscope, which uses optical sectioning to get better resolution of the fluorescence image.

Two-photon excitation microscopy is a fluorescence imaging technique that is particularly well-suited to image scattering living tissue of up to about one millimeter in thickness. Unlike traditional fluorescence microscopy, where the excitation wavelength is shorter than the emission wavelength, two-photon excitation requires simultaneous excitation by two photons with longer wavelength than the emitted light. The laser is focused onto a specific location in the tissue and scanned across the sample to sequentially produce the image. Due to the non-linearity of two-photon excitation, mainly fluorophores in the micrometer-sized focus of the laser beam are excited, which results in the spatial resolution of the image. This contrasts with confocal microscopy, where the spatial resolution is produced by the interaction of excitation focus and the confined detection with a pinhole.

ImageJ is a Java-based image processing program developed at the National Institutes of Health and the Laboratory for Optical and Computational Instrumentation. Its first version, ImageJ 1.x, is developed in the public domain, while ImageJ2 and the related projects SciJava, ImgLib2, and SCIFIO are licensed with a permissive BSD-2 license. ImageJ was designed with an open architecture that provides extensibility via Java plugins and recordable macros. Custom acquisition, analysis and processing plugins can be developed using ImageJ's built-in editor and a Java compiler. User-written plugins make it possible to solve many image processing and analysis problems, from three-dimensional live-cell imaging to radiological image processing, multiple imaging system data comparisons to automated hematology systems. ImageJ's plugin architecture and built-in development environment has made it a popular platform for teaching image processing.

Near-field scanning optical microscopy (NSOM) or scanning near-field optical microscopy (SNOM) is a microscopy technique for nanostructure investigation that breaks the far field resolution limit by exploiting the properties of evanescent waves. In SNOM, the excitation laser light is focused through an aperture with a diameter smaller than the excitation wavelength, resulting in an evanescent field on the far side of the aperture. When the sample is scanned at a small distance below the aperture, the optical resolution of transmitted or reflected light is limited only by the diameter of the aperture. In particular, lateral resolution of 6 nm and vertical resolution of 2–5 nm have been demonstrated.
CellProfiler is free, open-source software designed to enable biologists without training in computer vision or programming to quantitatively measure phenotypes from thousands of images automatically. Advanced algorithms for image analysis are available as individual modules that can be placed in sequential order together to form a pipeline; the pipeline is then used to identify and measure biological objects and features in images, particularly those obtained through fluorescence microscopy.
John Graham White is an Emeritus Professor of Anatomy and Molecular Biology at the University of Wisconsin–Madison. His research interests are in the biology of the model organism Caenorhabditis elegans and laser microscopy.

Second-harmonic imaging microscopy (SHIM) is based on a nonlinear optical effect known as second-harmonic generation (SHG). SHIM has been established as a viable microscope imaging contrast mechanism for visualization of cell and tissue structure and function. A second-harmonic microscope obtains contrasts from variations in a specimen's ability to generate second-harmonic light from the incident light while a conventional optical microscope obtains its contrast by detecting variations in optical density, path length, or refractive index of the specimen. SHG requires intense laser light passing through a material with a noncentrosymmetric molecular structure, either inherent or induced externally, for example by an electric field.
Winfried Denk is a German physicist. He built the first two-photon microscope while he was a graduate student in Watt W. Webb's lab at Cornell University, in 1989.

Xiaoliang Sunney Xie is a Chinese biophysicist well known for his contributions to the fields of single-molecule biophysical chemistry, coherent Raman Imaging and single-molecule genomics. In 2023, Xie renounced his U.S. citizenship in order to reclaim his Chinese citizenship.
Super-resolution microscopy is a series of techniques in optical microscopy that allow such images to have resolutions higher than those imposed by the diffraction limit, which is due to the diffraction of light. Super-resolution imaging techniques rely on the near-field or on the far-field. Among techniques that rely on the latter are those that improve the resolution only modestly beyond the diffraction-limit, such as confocal microscopy with closed pinhole or aided by computational methods such as deconvolution or detector-based pixel reassignment, the 4Pi microscope, and structured-illumination microscopy technologies such as SIM and SMI.

Martin Gruebele is a German-born American physical chemist and biophysicist who is currently James R. Eiszner Professor of Chemistry, Professor of Physics, Professor of Biophysics and Computational Biology at the University of Illinois Urbana-Champaign, where he is the principal investigator of the Gruebele Group.
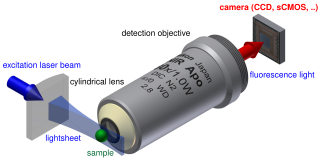
Light sheet fluorescence microscopy (LSFM) is a fluorescence microscopy technique with an intermediate-to-high optical resolution, but good optical sectioning capabilities and high speed. In contrast to epifluorescence microscopy only a thin slice of the sample is illuminated perpendicularly to the direction of observation. For illumination, a laser light-sheet is used, i.e. a laser beam which is focused only in one direction. A second method uses a circular beam scanned in one direction to create the lightsheet. As only the actually observed section is illuminated, this method reduces the photodamage and stress induced on a living sample. Also the good optical sectioning capability reduces the background signal and thus creates images with higher contrast, comparable to confocal microscopy. Because light sheet fluorescence microscopy scans samples by using a plane of light instead of a point, it can acquire images at speeds 100 to 1,000 times faster than those offered by point-scanning methods.

Robert Eric Betzig is an American physicist who works as a professor of physics and professor of molecular and cell biology at the University of California, Berkeley. He is also a senior fellow at the Janelia Farm Research Campus in Ashburn, Virginia.
Tip-enhanced Raman spectroscopy (TERS) is a variant of surface-enhanced Raman spectroscopy (SERS) that combines scanning probe microscopy with Raman spectroscopy. High spatial resolution chemical imaging is possible via TERS, with routine demonstrations of nanometer spatial resolution under ambient laboratory conditions, or better at ultralow temperatures and high pressure.

Stephen A. Boppart is a principal investigator at the Beckman Institute for Advanced Science and Technology at the University of Illinois at Urbana-Champaign, where he holds an Abel Bliss Professorship in engineering. He is a faculty member in the departments of electrical and computer engineering, bioengineering, and internal medicine. His research focus is biophotonics, where he has pioneered new optical imaging technologies in the fields of optical coherence tomography, multi-photon microscopy, and computational imaging.

Laura Ann Waller is a computer scientist and Ted Van Duzer Endowed Associate Professor at the University of California, Berkeley. She was awarded a Chan Zuckerberg Initiative Fellowship to develop microscopes to image deep structures within the brain in 2017 and won the 2018 SPIE Early Career Award.
John Marius Rodenburg is Professor in the Department of Electronic and Electrical Engineering at the University of Sheffield.
Melissa Caroline Skala is an American biomedical engineer who is a professor at the Morgridge Institute for Research. Her research considers photonics-based technologies for personalised medical therapies. She is a Fellow of The Optical Society, SPIE and American Institute for Medical and Biological Engineering.
Katrina T. Forest is an American biologist who is the EB Fred Professor of Bacteriology and Chair in the Department of Bacteriology at the University of Wisconsin–Madison. Her research considers the use of structural biology to better understand pathogenesis. Forest is a Fellow of the American Society for Microbiology.

Wibool Piyawattanametha is the head of Advanced Imaging Research (AIR) Center, King Mongkut's Institute of Technology Ladkrabang, Thailand.











