Related Research Articles
The Mössbauer effect, or recoilless nuclear resonance fluorescence, is a physical phenomenon discovered by Rudolf Mössbauer in 1958. It involves the resonant and recoil-free emission and absorption of gamma radiation by atomic nuclei bound in a solid. Its main application is in Mössbauer spectroscopy.

The neutrinoless double beta decay (0νββ) is a commonly proposed and experimentally pursued theoretical radioactive decay process that would prove a Majorana nature of the neutrino particle. To this day, it has not been found.

In particle physics, the history of quantum field theory starts with its creation by Paul Dirac, when he attempted to quantize the electromagnetic field in the late 1920s. Heisenberg was awarded the 1932 Nobel Prize in Physics "for the creation of quantum mechanics". Major advances in the theory were made in the 1940s and 1950s, leading to the introduction of renormalized quantum electrodynamics (QED). QED was so successful and accurately predictive that efforts were made to apply the same basic concepts for the other forces of nature. By the late 1970s, these efforts successfully utilized gauge theory in the strong nuclear force and weak nuclear force, producing the modern Standard Model of particle physics.
Thorium (90Th) has seven naturally occurring isotopes but none are stable. One isotope, 232Th, is relatively stable, with a half-life of 1.405×1010 years, considerably longer than the age of the Earth, and even slightly longer than the generally accepted age of the universe. This isotope makes up nearly all natural thorium, so thorium was considered to be mononuclidic. However, in 2013, IUPAC reclassified thorium as binuclidic, due to large amounts of 230Th in deep seawater. Thorium has a characteristic terrestrial isotopic composition and thus a standard atomic weight can be given.
Radium (88Ra) has no stable or nearly stable isotopes, and thus a standard atomic weight cannot be given. The longest lived, and most common, isotope of radium is 226Ra with a half-life of 1600 years. 226Ra occurs in the decay chain of 238U. Radium has 34 known isotopes from 201Ra to 234Ra.
Natural tantalum (73Ta) consists of two stable isotopes: 181Ta (99.988%) and 180m
Ta
(0.012%).
There are 39 known isotopes and 17 nuclear isomers of tellurium (52Te), with atomic masses that range from 104 to 142. These are listed in the table below.
Americium (95Am) is an artificial element, and thus a standard atomic weight cannot be given. Like all artificial elements, it has no known stable isotopes. The first isotope to be synthesized was 241Am in 1944. The artificial element decays by ejecting alpha particles. Americium has an atomic number of 95. Despite 243
Am being an order of magnitude longer lived than 241
Am, the former is harder to obtain than the latter as more of it is present in spent nuclear fuel.
Curium (96Cm) is an artificial element with an atomic number of 96. Because it is an artificial element, a standard atomic weight cannot be given, and it has no stable isotopes. The first isotope synthesized was 242Cm in 1944, which has 146 neutrons.
Berkelium (97Bk) is an artificial element, and thus a standard atomic weight cannot be given. Like all artificial elements, it has no stable isotopes. The first isotope to be synthesized was 243Bk in 1949. There are 20 known radioisotopes, from 230Bk and 233Bk to 253Bk, and 6 nuclear isomers. The longest-lived isotope is 247Bk with a half-life of 1,380 years.
Dubnium (105Db) is a synthetic element, thus a standard atomic weight cannot be given. Like all synthetic elements, it has no stable isotopes. The first isotope to be synthesized was 261Db in 1968. The 13 known radioisotopes are from 255Db to 270Db, and 1–3 isomers. The longest-lived known isotope is 268Db with a half-life of 16 hours.

Tony Hilton Royle Skyrme was a British physicist.

In nuclear and materials physics, stopping power is the retarding force acting on charged particles, typically alpha and beta particles, due to interaction with matter, resulting in loss of particle kinetic energy. Its application is important in areas such as radiation protection, ion implantation and nuclear medicine.
In physics, quasielastic scattering designates a limiting case of inelastic scattering, characterized by energy transfers being small compared to the incident energy of the scattered particles.
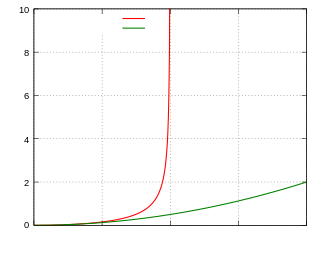
Tests of relativistic energy and momentum are aimed at measuring the relativistic expressions for energy, momentum, and mass. According to special relativity, the properties of particles moving approximately at the speed of light significantly deviate from the predictions of Newtonian mechanics. For instance, the speed of light cannot be reached by massive particles.

Tom Wilkerson Bonner was an American experimental physicist who developed important instruments and techniques for neutron physics and nuclear physics.
Several hundred metals, compounds, alloys and ceramics possess the property of superconductivity at low temperatures. The SU(2) color quark matter adjoins the list of superconducting systems. Although it is a mathematical abstraction, its properties are believed to be closely related to the SU(3) color quark matter, which exists in nature when ordinary matter is compressed at supranuclear densities above ~ 0.5 1039 nucleon/cm3.
George Raymond "Ray" Satchler was a British-American nuclear physicist.
Felix Hans Boehm was a Swiss-American experimental physicist, known for his research on weak interactions, parity violation, and neutrino physics.
Henry Winston Newson was an American physical chemist and nuclear physicist, known for his research on nuclear resonances and as one of the co-inventors of the control system used in nuclear reactors.
References
- ↑ "Lemuel Wyly Jr". Legacy. Retrieved 10 July 2019.
- ↑ Avignone, Frank T.; Braden, C.H.; Patronis, E.T.; Wyly, L.D. (1966). "Conversion-electron-gamma directional correlation in 133Cs". Nuclear Physics. 80 (2): 314–320. doi:10.1016/0029-5582(66)90091-5.
- ↑ Dulaney, Harry; Braden, C.H.; Wyly, L.D. (1964). "Investigation of the nuclear matrix element parameters for first-forbidden beta decay". Nuclear Physics. 52: 79–92. doi:10.1016/0029-5582(64)90676-5.
- ↑ Wyly, L. D. (1949). "Angular Distribution of Protons in theN14(dp)N15Reaction". Physical Review. 76: 104–106. doi:10.1103/PhysRev.76.104.
- ↑ "Wyly, L. D. - The Online Books Page". upenn.edu. Retrieved 8 May 2016.
- ↑ "Estelle Wyly Obituary - Stone Mountain, GA - Atlanta Journal-Constitution". Atlanta Journal-Constitution. Retrieved 8 May 2016.
- http://onlinebooks.library.upenn.edu/webbin/book/lookupname?key=Wyly%2C%20L.%20D.
- https://catalog.hathitrust.org/Record/011572027
- Avignone, Frank T.; Braden, C.H.; Patronis, E.T.; Wyly, L.D. (1966). "Conversion-electron-gamma directional correlation in 133Cs". Nuclear Physics. 80 (2): 314–320. doi:10.1016/0029-5582(66)90091-5.
- https://journals.aps.org/pr/abstract/10.1103/PhysRev.76.104