Related Research Articles

A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those that include transition metals, are coordination complexes.

Cis–trans isomerism, also known as geometric isomerism, describes certain arrangements of atoms within molecules. The prefixes "cis" and "trans" are from Latin: "this side of" and "the other side of", respectively. In the context of chemistry, cis indicates that the functional groups (substituents) are on the same side of some plane, while trans conveys that they are on opposing (transverse) sides. Cis–trans isomers are stereoisomers, that is, pairs of molecules which have the same formula but whose functional groups are in different orientations in three-dimensional space. Cis and trans isomers occur both in organic molecules and in inorganic coordination complexes. Cis and trans descriptors are not used for cases of conformational isomerism where the two geometric forms easily interconvert, such as most open-chain single-bonded structures; instead, the terms "syn" and "anti" are used.

In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands".

Alfred Werner was a Swiss chemist who was a student at ETH Zurich and a professor at the University of Zurich. He won the Nobel Prize in Chemistry in 1913 for proposing the octahedral configuration of transition metal complexes. Werner developed the basis for modern coordination chemistry. He was the first inorganic chemist to win the Nobel Prize, and the only one prior to 1973.

Bipyridines are a family of organic compounds with the formula (C5H4N)2, consisting of two pyridyl (C5H4N) rings. Pyridine is an aromatic nitrogen-containing heterocycle. The bipyridines are all colourless solids, which are soluble in organic solvents and slightly soluble in water. Bipyridines, especially the 4,4' isomer, are mainly of significance in pesticides.
In chemistry, linkage isomerism or ambidentate isomerism is a form of isomerism in which certain coordination compounds have the same composition but differ in their metal atom's connectivity to a ligand.

In chemistry, octahedral molecular geometry, also called square bipyramidal, describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The octahedron has eight faces, hence the prefix octa. The octahedron is one of the Platonic solids, although octahedral molecules typically have an atom in their centre and no bonds between the ligand atoms. A perfect octahedron belongs to the point group Oh. Examples of octahedral compounds are sulfur hexafluoride SF6 and molybdenum hexacarbonyl Mo(CO)6. The term "octahedral" is used somewhat loosely by chemists, focusing on the geometry of the bonds to the central atom and not considering differences among the ligands themselves. For example, [Co(NH3)6]3+, which is not octahedral in the mathematical sense due to the orientation of the N−H bonds, is referred to as octahedral.

In coordination chemistry, metal ammine complexes are metal complexes containing at least one ammonia ligand. "Ammine" is spelled this way for historical reasons; in contrast, alkyl or aryl bearing ligands are spelt with a single "m". Almost all metal ions bind ammonia as a ligand, but the most prevalent examples of ammine complexes are for Cr(III), Co(III), Ni(II), Cu(II) as well as several platinum group metals.
Coordination isomerism is a form of structural isomerism in which the composition of the coordination complex ion varies. In a coordination isomer the total ratio of ligand to metal remains the same, but the ligands attached to a specific metal ion change. Examples of a complete series of coordination isomers require at least two metal ions and sometimes more.

In coordination chemistry, the bite angle is the angle on a central atom between two bonds to a bidentate ligand. This ligand–metal–ligand geometric parameter is used to classify chelating ligands, including those in organometallic complexes. It is most often discussed in terms of catalysis, as changes in bite angle can affect not just the activity and selectivity of a catalytic reaction but even allow alternative reaction pathways to become accessible.
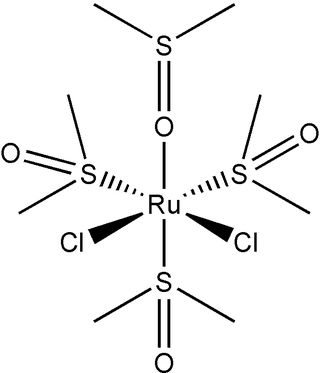
Dichlorotetrakis(dimethyl sulfoxide) ruthenium(II) describes coordination compounds with the formula RuCl2(dmso)4, where DMSO is dimethylsulfoxide. Both cis and trans isomers are known, but the cis isomer is more common. The cis isomer is a yellow, air-stable solid that is soluble in some organic solvents. These sulfoxide complexes are used in the synthesis of various ruthenium(ii) complexes. They have also attracted attention as possible anti-cancer drugs.

In coordination chemistry, denticity refers to the number of donor groups in a given ligand that bind to the central metal atom in a coordination complex. In many cases, only one atom in the ligand binds to the metal, so the denticity equals one, and the ligand is said to be monodentate. Ligands with more than one bonded atom are called polydentate or multidentate. The denticity of a ligand is described with the Greek letter κ ('kappa'). For example, κ6-EDTA describes an EDTA ligand that coordinates through 6 non-contiguous atoms.

Nickel(II) bis(acetylacetonate) is a coordination complex with the formula [Ni(acac)2]3, where acac is the anion C5H7O2− derived from deprotonation of acetylacetone. It is a dark green paramagnetic solid that is soluble in organic solvents such as toluene. It reacts with water to give the blue-green diaquo complex Ni(acac)2(H2O)2.

1,2-Diaminopropane (propane-1,2-diamine) is organic compound with the formula CH3CH(NH2)CH2NH2. A colorless liquid, it is the simplest chiral diamine. It is used as a bidentate ligand in coordination chemistry.
In chemistry, tetradentate ligands are ligands that bind four donor atoms to a central atom to form a coordination complex. This number of donor atoms that bind is called denticity and is a method of classifying ligands.

A tridentate ligand is a ligand that has three atoms that can function as donor atoms in a coordination complex.

Salpn is the common name for a chelating ligand, properly called N,N′-bis(salicylidene)-1,2-propanediamine, used as a motor oil additive.
Transition metal amino acid complexes are a large family of coordination complexes containing the conjugate bases of the amino acids, the 2-aminocarboxylates. Amino acids are prevalent in nature, and all of them function as ligands toward the transition metals. Not included in this article are complexes of the amides and ester derivatives of amino acids. Also excluded are the polyamino acids including the chelating agents EDTA and NTA.

In organometallic chemistry, transition metal complexes of nitrite describes families of coordination complexes containing one or more nitrite ligands. Although the synthetic derivatives are only of scholarly interest, metal-nitrite complexes occur in several enzymes that participate in the nitrogen cycle.
Cobalt compounds are chemical compounds formed by cobalt with other elements.
References
- ↑ "24.4: Isomerism in Coordination Complexes". Chemistry LibreTexts. 2015-01-18. Retrieved 2024-08-23.
- ↑ UCI Department of Chemistry (13 November 2015). "Coordination Chemistry I: Structures and Isomer" (PDF).