
The lipid bilayer is a thin polar membrane made of two layers of lipid molecules. These membranes are flat sheets that form a continuous barrier around all cells. The cell membranes of almost all organisms and many viruses are made of a lipid bilayer, as are the nuclear membrane surrounding the cell nucleus, and membranes of the membrane-bound organelles in the cell. The lipid bilayer is the barrier that keeps ions, proteins and other molecules where they are needed and prevents them from diffusing into areas where they should not be. Lipid bilayers are ideally suited to this role, even though they are only a few nanometers in width, because they are impermeable to most water-soluble (hydrophilic) molecules. Bilayers are particularly impermeable to ions, which allows cells to regulate salt concentrations and pH by transporting ions across their membranes using proteins called ion pumps.

A transmembrane protein (TP) is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequently undergo significant conformational changes to move a substance through the membrane. They are usually highly hydrophobic and aggregate and precipitate in water. They require detergents or nonpolar solvents for extraction, although some of them (beta-barrels) can be also extracted using denaturing agents.

Photosystem II is the first protein complex in the light-dependent reactions of oxygenic photosynthesis. It is located in the thylakoid membrane of plants, algae, and cyanobacteria. Within the photosystem, enzymes capture photons of light to energize electrons that are then transferred through a variety of coenzymes and cofactors to reduce plastoquinone to plastoquinol. The energized electrons are replaced by oxidizing water to form hydrogen ions and molecular oxygen.

Lipid A is a lipid component of an endotoxin held responsible for the toxicity of gram-negative bacteria. It is the innermost of the three regions of the lipopolysaccharide (LPS), also called endotoxin molecule, and its hydrophobic nature allows it to anchor the LPS to the outer membrane. While its toxic effects can be damaging, the sensing of lipid A by the human immune system may also be critical for the onset of immune responses to gram-negative infection, and for the subsequent successful fight against the infection.
Alkylresorcinols, also known as resorcinolic lipids, are phenolic lipids composed of long aliphatic chains and resorcinol-type phenolic rings.

The bacterial outer membrane is found in gram-negative bacteria. Its composition is distinct from that of the inner cytoplasmic cell membrane - among other things, the outer leaflet of the outer membrane of many gram-negative bacteria includes a complex lipopolysaccharide whose lipid portion acts as an endotoxin - and in some bacteria such as E. coli it is linked to the cell's peptidoglycan by Braun's lipoprotein.

Hexosaminidase is an enzyme involved in the hydrolysis of terminal N-acetyl-D-hexosamine residues in N-acetyl-β-D-hexosaminides.
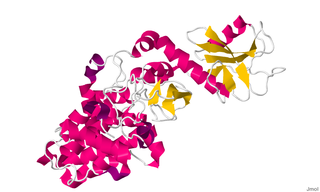
Chimerin 1, (CHN1) also known as alpha-1-chimerin, n-chimerin is a protein which in humans is encoded by the CHN1 gene.

Glycerol-3-phosphate dehydrogenase (GPDH) is an enzyme that catalyzes the reversible redox conversion of dihydroxyacetone phosphate to sn-glycerol 3-phosphate.
Ceramidase is an enzyme which cleaves fatty acids from ceramide, producing sphingosine (SPH) which in turn is phosphorylated by a sphingosine kinase to form sphingosine-1-phosphate (S1P).
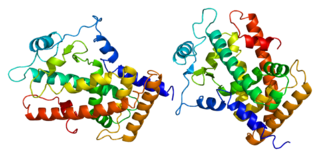
Peroxisome proliferator-activated receptor beta or delta, also known as NR1C2 is a nuclear receptor that in humans is encoded by the PPARD gene.
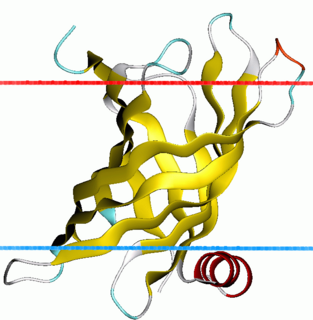
Antimicrobial peptide resistance and lipid A acylation protein PagP is a family of several bacterial antimicrobial peptide resistance and lipid A acylation (PagP) proteins. The bacterial outer membrane enzyme PagP transfers a palmitate chain from a phospholipid to lipid A. In a number of pathogenic Gram-negative bacteria, PagP confers resistance to certain cationic antimicrobial peptides produced during the host innate immune response.

Flotillin-1 is a protein that in humans is encoded by the FLOT1 gene.

Tricarboxylate transport protein, mitochondrial, also known as tricarboxylate carrier protein and citrate transport protein (CTP), is a protein that in humans is encoded by the SLC25A1 gene. SLC25A1 belongs to the mitochondrial carrier gene family SLC25. High levels of the tricarboxylate transport protein are found in the liver, pancreas and kidney. Lower or no levels are present in the brain, heart, skeletal muscle, placenta and lung.
Archaea constitute a domain of single-celled organisms. These microorganisms lack cell nuclei and are therefore prokaryotes. Archaea were initially classified as bacteria, receiving the name archaebacteria, but this term has fallen out of use.
N-Acylphosphatidylethanolamines (NAPEs) are hormones released by the small intestine into the bloodstream when it processes fat. NAPEs travel to the hypothalamus in the brain and suppress appetite. This mechanism could be relevant for treating obesity.
Mechanosensitive channels, mechanosensitive ion channels or stretch-gated ion channels (not to be confused with mechanoreceptors). They are present in the membranes of organisms from the three domains of life: bacteria, archaea, and eukarya. They are the sensors for a number of systems including the senses of touch, hearing and balance, as well as participating in cardiovascular regulation and osmotic homeostasis (e.g. thirst). The channels vary in selectivity for the permeating ions from nonselective between anions and cations in bacteria, to cation selective allowing passage Ca2+, K+ and Na+ in eukaryotes, and highly selective K+ channels in bacteria and eukaryotes.

Abnormal cannabidiol (Abn-CBD) is a synthetic regioisomer of cannabidiol, which unlike most other cannabinoids produces vasodilator effects, lowers blood pressure, and induces cell migration, cell proliferation and mitogen-activated protein kinase activation in microglia, but without producing any psychoactive effects.

The Saffman–Delbrück model describes a lipid membrane as a thin layer of viscous fluid, surrounded by a less viscous bulk liquid. This picture was originally proposed to determine the diffusion coefficient of membrane proteins, but has also been used to describe the dynamics of fluid domains within lipid membranes. The Saffman–Delbrück formula is often applied to determine the size of an object embedded in a membrane from its observed diffusion coefficient, and is characterized by the weak logarithmic dependence of diffusion constant on object radius.

Methionine sulfoxide is the organic compound with the formula CH3S(O)CH2CH2CH(NH2)CO2H. It is an amino acid that occurs naturally although it is formed post-translationally.
















