
Sulfuric acid (alternative spelling sulphuric acid), also known as vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with molecular formula H2SO4. It is a colorless, odorless, and syrupy liquid that is soluble in water, in a reaction that is highly exothermic.

A detonator, frequently a blasting cap, is a device used to trigger an explosive device. Detonators can be chemically, mechanically, or electrically initiated, the latter two being the most common.
A hydrometer is an instrument used for measuring the relative density of liquids based on the concept of buoyancy. They are typically calibrated and graduated with one or more scales such as specific gravity.
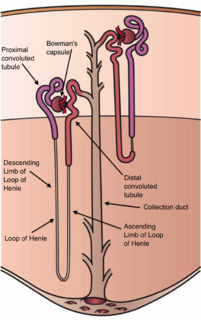
The nephron is the microscopic structural and functional unit of the kidney. It is composed of a renal corpuscle and a renal tubule. The renal corpuscle consists of a tuft of capillaries called a glomerulus and an encompassing Bowman's capsule. The renal tubule extends from the capsule. The capsule and tubule are connected and are composed of epithelial cells with a lumen. A healthy adult has 0.8 to 1.5 million nephrons in each kidney. Blood is filtered as it passes through three layers: the endothelial cells of the capillary wall, its basement membrane, and between the foot processes of the podocytes of the lining of the capsule. The tubule has adjacent peritubular capillaries that run between the descending and ascending portions of the tubule. As the fluid from the capsule flows down into the tubule, it is processed by the epithelial cells lining the tubule: water is reabsorbed and substances are exchanged ; first with the interstitial fluid outside the tubules, and then into the plasma in the adjacent peritubular capillaries through the endothelial cells lining that capillary. This process regulates the volume of body fluid as well as levels of many body substances. At the end of the tubule, the remaining fluid—urine—exits: it is composed of water, metabolic waste, and toxins.

A gas-filled tube, also known as a discharge tube, is an arrangement of electrodes in a gas within an insulating, temperature-resistant envelope. Gas-filled tubes exploit phenomena related to electric discharge in gases, and operate by ionizing the gas with an applied voltage sufficient to cause electrical conduction by the underlying phenomena of the Townsend discharge. A gas-discharge lamp is an electric light using a gas-filled tube; these include fluorescent lamps, metal-halide lamps, sodium-vapor lamps, and neon lights. Specialized gas-filled tubes such as krytrons, thyratrons, and ignitrons are used as switching devices in electric devices.

Intestinal villi are small, finger-like projections that extend into the lumen of the small intestine. Each villus is approximately 0.5–1.6 mm in length, and has many microvilli projecting from the enterocytes of its epithelium which collectively form the striated or brush border. Each of these microvilli are much smaller than a single villus. The intestinal villi are much smaller than any of the circular folds in the intestine.

Anodizing is an electrolytic passivation process used to increase the thickness of the natural oxide layer on the surface of metal parts.

A McLeod gauge is a scientific instrument used to measure very low pressures, down to 10−6 Torr. It was invented in 1874 by Herbert McLeod (1841–1923). McLeod gauges were once commonly found attached to equipment that operates under vacuum, such as a lyophilizer. Today, however, these gauges have largely been replaced by electronic vacuum gauges.

A mercury coulometer is an electroanalytical chemistry device using mercury to determine the amount of matter transformed during the following reaction:

Thin-layer chromatography (TLC) is a chromatography technique used to separate non-volatile mixtures. Thin-layer chromatography is performed on a sheet of glass, plastic, or aluminium foil, which is coated with a thin layer of adsorbent material, usually silica gel, aluminium oxide (alumina), or cellulose. This layer of adsorbent is known as the stationary phase.

James Marsh was a British chemist who invented the Marsh test for detecting arsenic.

Six's thermometer is a registering thermometer which can record the maximum and minimum temperatures reached over a period of time, for example 24 hours. It is used to record the extremes of temperature at a location, for instance in meteorology and horticulture. It was invented by a British scientist James Six, born in Canterbury, in 1780; the same basic design remains in use.

Kipp's apparatus, also called Kipp generator, is an apparatus designed for preparation of small volumes of gases. It was invented around 1844 by the Dutch pharmacist Petrus Jacobus Kipp and widely used in chemical laboratories and for demonstrations in schools into the second half of the 20th century.

Gaston Planté was the French physicist who invented the lead–acid battery in 1859. The lead-acid battery eventually became the first rechargeable electric battery marketed for commercial use and is widely used in automobiles.

A voltameter or coulometer is a scientific instrument used for measuring quantity of electricity through electrolytic action. The SI unit of quantity of electricity is the coulomb.

The Zamboni pile is an early electric battery, invented by Giuseppe Zamboni in 1812.
The Sprengel zinti pump is a vacuum pump that uses drops of mercury falling through a small-bore capillary tube to trap air from the system to be evacuated. It was invented by Hanover-born chemist Hermann Sprengel in 1865 while he was working in London. The pump created the highest vacuum achievable at that time, less than 1 mPa.
A pencil bomb was a type of time bomb with a timer that could be set to detonate any given time. It was designed by German chemist Dr. Walter Scheele and used by German spy Franz von Rintelen during World War I.




















