
A weight (also known as a mass) is an object, normally with high density, whose chief task is to have mass and exert weight (through gravity). It is used for different purposes, such as in:

A weight (also known as a mass) is an object, normally with high density, whose chief task is to have mass and exert weight (through gravity). It is used for different purposes, such as in:

The atomic number or nuclear charge number of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (np) or the number of protons found in the nucleus of every atom of that element. The atomic number can be used to uniquely identify ordinary chemical elements. In an ordinary uncharged atom, the atomic number is also equal to the number of electrons.

Body mass index (BMI) is a value derived from the mass (weight) and height of a person. The BMI is defined as the body mass divided by the square of the body height, and is expressed in units of kg/m2, resulting from mass in kilograms (kg) and height in metres (m).

The kilogram is the base unit of mass in the International System of Units (SI), having the unit symbol kg. 'Kilogram' means 'one thousand grams' and is colloquially abbreviated to kilo.

Mass is an intrinsic property of a body. It was traditionally believed to be related to the quantity of matter in a body, until the discovery of the atom and particle physics. It was found that different atoms and different elementary particles, theoretically with the same amount of matter, have nonetheless different masses. Mass in modern physics has multiple definitions which are conceptually distinct, but physically equivalent. Mass can be experimentally defined as a measure of the body's inertia, meaning the resistance to acceleration when a net force is applied. The object's mass also determines the strength of its gravitational attraction to other bodies.
The molecular mass is the mass of a given molecule. Units of daltons (Da) are often used. Different molecules of the same compound may have different molecular masses because they contain different isotopes of an element. The derived quantity relative molecular mass is the unitless ratio of the mass of a molecule to the atomic mass constant.

The pound or pound-mass is a unit of mass used in both the British imperial and United States customary systems of measurement. Various definitions have been used; the most common today is the international avoirdupois pound, which is legally defined as exactly 0.45359237 kilograms, and which is divided into 16 avoirdupois ounces. The international standard symbol for the avoirdupois pound is lb; an alternative symbol is lbm, #, and ℔ or ″̶.
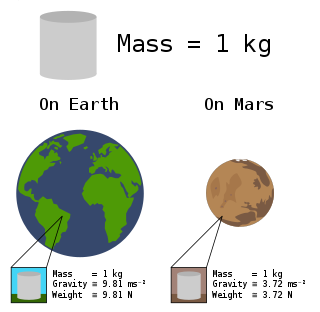
In science and engineering, the weight of an object is a quantity associated with the gravitational force exerted on the object by other objects in its environment, although there is some variation and debate as to the exact definition.

The mole (symbol mol) is a unit of measurement, the base unit in the International System of Units (SI) for amount of substance, a quantity proportional to the number of elementary entities of a substance. One mole is an aggregate of exactly 6.02214076×1023 elementary entities (approximately 602 sextillion or 602 billion times a trillion), which can be atoms, molecules, ions, ion pairs, or other particles. The number of particles in a mole is the Avogadro number (symbol N0) and the numerical value of the Avogadro constant (symbol NA) expressed in mol-1. The value was chosen on the basis of the historical definition of the mole as the amount of substance that corresponds to the number of atoms in 12 grams of 12C, which made the mass of a mole of a compound expressed in grams, numerically equal to the average molecular mass or formula mass of the compound expressed in daltons. With the 2019 revision of the SI, the numerical equivalence is now only approximate but may be assumed for all practical purposes.
The dalton or unified atomic mass unit is a unit of mass defined as 1/12 of the mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state and at rest. It is a non-SI unit accepted for use with SI. The atomic mass constant, denoted mu, is defined identically, giving mu = 1/12m(12C) = 1 Da.

A pendulum is a device made of a weight suspended from a pivot so that it can swing freely. When a pendulum is displaced sideways from its resting, equilibrium position, it is subject to a restoring force due to gravity that will accelerate it back toward the equilibrium position. When released, the restoring force acting on the pendulum's mass causes it to oscillate about the equilibrium position, swinging back and forth. The time for one complete cycle, a left swing and a right swing, is called the period. The period depends on the length of the pendulum and also to a slight degree on the amplitude, the width of the pendulum's swing.
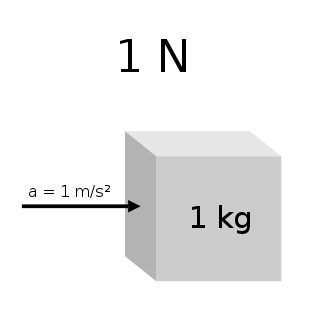
The newton is the unit of force in the International System of Units (SI). Expressed in terms of SI base units, it is 1 kg⋅m/s2, the force that accelerates a mass of one kilogram at one metre per second squared.

In chemistry, the molar mass of a chemical compound is defined as the ratio between the mass and the amount of substance of any sample of the compound. The molar mass is a bulk, not molecular, property of a substance. The molar mass is an average of many instances of the compound, which often vary in mass due to the presence of isotopes. Most commonly, the molar mass is computed from the standard atomic weights and is thus a terrestrial average and a function of the relative abundance of the isotopes of the constituent atoms on Earth. The molar mass is appropriate for converting between the mass of a substance and the amount of a substance for bulk quantities.
Relative atomic mass, also known by the deprecated synonym atomic weight, is a dimensionless physical quantity defined as the ratio of the average mass of atoms of a chemical element in a given sample to the atomic mass constant. The atomic mass constant is defined as being 1/12 of the mass of a carbon-12 atom. Since both quantities in the ratio are masses, the resulting value is dimensionless. These definitions remain valid even after the 2019 revision of the SI.

The mass number (symbol A, from the German word: Atomgewicht, "atomic weight"), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units. Since protons and neutrons are both baryons, the mass number A is identical with the baryon number B of the nucleus (and also of the whole atom or ion). The mass number is different for each isotope of a given chemical element, and the difference between the mass number and the atomic number Z gives the number of neutrons (N) in the nucleus: N = A − Z.

A scale or balance is a device used to measure weight or mass. These are also known as mass scales, weight scales, mass balances, massometers, and weight balances.
A bob is a heavy object on the end of a pendulum found most commonly, but not exclusively, in pendulum clocks.

The standard atomic weight of a chemical element (symbol Ar°(E) for element "E") is the weighted arithmetic mean of the relative isotopic masses of all isotopes of that element weighted by each isotope's abundance on Earth. For example, isotope 63Cu (Ar = 62.929) constitutes 69% of the copper on Earth, the rest being 65Cu (Ar = 64.927), so

A Wilberforce pendulum, invented by British physicist Lionel Robert Wilberforce around 1896, consists of a mass suspended by a long helical spring and free to turn on its vertical axis, twisting the spring. It is an example of a coupled mechanical oscillator, often used as a demonstration in physics education. The mass can both bob up and down on the spring, and rotate back and forth about its vertical axis with torsional vibrations. When correctly adjusted and set in motion, it exhibits a curious motion in which periods of purely rotational oscillation gradually alternate with periods of purely up and down oscillation. The energy stored in the device shifts slowly back and forth between the translational 'up and down' oscillation mode and the torsional 'clockwise and counterclockwise' oscillation mode, until the motion eventually dies away.
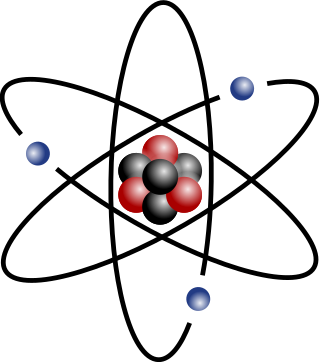
Atomic mass (ma or m) is the mass of a single atom. The atomic mass mostly comes from the combined mass of the protons and neutrons in the nucleus, with minor contributions from the electrons and nuclear binding energy. The atomic mass of atoms, ions, or atomic nuclei is slightly less than the sum of the masses of their constituent protons, neutrons, and electrons, due to (per E = mc2).
Vehicle weight is a measurement of wheeled motor vehicles; either an actual measured weight of the vehicle under defined conditions or a gross weight rating for its weight carrying capacity.