Related Research Articles

X-ray fluorescence (XRF) is the emission of characteristic "secondary" X-rays from a material that has been excited by being bombarded with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science, archaeology and art objects such as paintings

A perovskite is any material with the same type of crystal structure as calcium titanium oxide (CaTiO3), known as the perovskite structure. Perovskites take their name from the mineral, which was first discovered in the Ural mountains of Russia by Gustav Rose in 1839 and is named after Russian mineralogist L. A. Perovski (1792–1856). The general chemical formula for perovskite compounds is ABX3, where 'A' and 'B' are two cations of very different sizes, and X is an anion that bonds to both. The 'A' atoms are larger than the 'B' atoms. The ideal cubic structure has the B cation in 6-fold coordination, surrounded by an octahedron of anions, and the A cation in 12-fold cuboctahedral coordination.

A scintillator is a material that exhibits scintillation, the property of luminescence, when excited by ionizing radiation. Luminescent materials, when struck by an incoming particle, absorb its energy and scintillate. Sometimes, the excited state is metastable, so the relaxation back down from the excited state to lower states is delayed. The process then corresponds to one of two phenomena: delayed fluorescence or phosphorescence. The correspondence depends on the type of transition and hence the wavelength of the emitted optical photon.
Wavelength-dispersive X-ray spectroscopy is a method used to count the number of X-rays of a specific wavelength diffracted by a crystal. The wavelength of the impinging X-ray and the crystal's lattice spacings are related by Bragg's law and produce constructive interference if they fit the criteria of Bragg's law. Unlike the related technique of energy-dispersive X-ray spectroscopy (EDS), WDS reads or counts only the X-rays of a single wavelength at a time, not producing a broad spectrum of wavelengths or energies simultaneously. WDS is primarily used in chemical analysis, in an X-ray fluorescence spectrometer, in an electron microprobe, and may also be used in a scanning electron microscope.
Scintillation is a flash of light produced in a transparent material by the passage of a particle. See scintillator and scintillation counter for practical applications.

Scheelite is a calcium tungstate mineral with the chemical formula CaWO4. It is an important ore of tungsten (wolfram). Well-formed crystals are sought by collectors and are occasionally fashioned into gemstones when suitably free of flaws. Scheelite has been synthesized using the Czochralski process; the material produced may be used to imitate diamond, as a scintillator, or as a solid-state lasing medium. It was also used in radium paint in the same fashion as was zinc sulphide, and Thomas Edison invented a fluoroscope with a calcium tungstate-coated screen, making the images six times brighter than those with barium platinocyanide; the latter chemical allowed Röntgen to discover X-rays in early November 1895.

Caesium iodide or cesium iodide is the ionic compound of caesium and iodine. It is often used as the input phosphor of an X-ray image intensifier tube found in fluoroscopy equipment. Caesium iodide photocathodes are highly efficient at extreme ultraviolet wavelengths.

Sodium iodide (chemical formula NaI) is an ionic compound formed from the chemical reaction of sodium metal and iodine. Under standard conditions, it is a white, water-soluble solid comprising a 1:1 mix of sodium cations (Na+) and iodide anions (I−) in a crystal lattice. It is used mainly as a nutritional supplement and in organic chemistry. It is produced industrially as the salt formed when acidic iodides react with sodium hydroxide. It is a chaotropic salt.
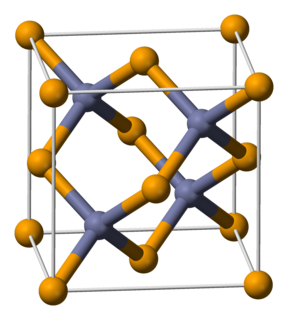
Zinc selenide (ZnSe) is a light-yellow, solid compound comprising zinc (Zn) and selenium (Se). It is an intrinsic semiconductor with a band gap of about 2.70 eV at 25 °C (77 °F). ZnSe rarely occurs in nature, and is found in the mineral that was named after Hans Stille called "stilleite."
Neutron detection is the effective detection of neutrons entering a well-positioned detector. There are two key aspects to effective neutron detection: hardware and software. Detection hardware refers to the kind of neutron detector used and to the electronics used in the detection setup. Further, the hardware setup also defines key experimental parameters, such as source-detector distance, solid angle and detector shielding. Detection software consists of analysis tools that perform tasks such as graphical analysis to measure the number and energies of neutrons striking the detector.

Stolzite is a mineral, a lead tungstate; with the formula PbWO4. It is similar to, and often associated with, wulfenite which is the same chemical formula except that the tungsten is replaced by molybdenum. Stolzite crystallizes in the tetragonal crystal system and is dimorphous with the monoclinic form raspite.

Bismuth germanium oxide or bismuth germanate is an inorganic chemical compound of bismuth, germanium and oxygen. Most commonly the term refers to the compound with chemical formula Bi4Ge3O12 (BGO), with the cubic evlitine crystal structure, used as a scintillator. (The term may also refer to a different compound with formula Bi12GeO20, an electro-optical material with sillenite structure, and Bi2Ge3O9.)
Cadmium tungstate (CdWO4 or CWO), the cadmium salt of tungstic acid, is a dense, chemically inert solid which is used as a scintillation crystal to detect gamma rays. It has density of 7.9 g/cm3 and melting point of 1325 °C. It is toxic if inhaled or swallowed. Its crystals are transparent, colorless, with slight yellow tint. It is odorless. Its CAS number is . It is not hygroscopic.
Lanthanum chloride is the inorganic compound with the formula LaCl3. It is a common salt of lanthanum which is mainly used in research. It is a white solid that is highly soluble in water and alcohols.
Lithium molybdate (Li2MoO4) is a chemical compound. It is mainly used as an inhibitor in some types of industrial air conditioning.
Gadolinium oxysulfide (Gd2O2S), also called gadolinium sulfoxylate, GOS or Gadox, is an inorganic compound, a mixed oxide-sulfide of gadolinium. Its CAS number is .
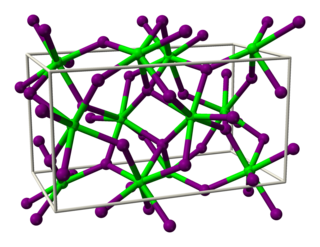
Strontium iodide (SrI2) is a salt of strontium and iodine. It is an ionic, water-soluble, and deliquescent compound that can be used in medicine as a substitute for potassium iodide . It is also used as a scintillation gamma radiation detector, typically doped with europium, due to its optical clarity, relatively high density, high effective atomic number (Z=48), and high scintillation light yield. In recent years, europium-doped strontium iodide (SrI2:Eu2+) has emerged as a promising scintillation material for gamma-ray spectroscopy with extremely high light yield and proportional response, exceeding that of the widely used high performance commercial scintillator LaBr3:Ce3+. Large diameter SrI2 crystals can be grown reliably using vertical Bridgman technique and are being commercialized by several companies.

Cerium(III) bromide is an inorganic compound with the formula CeBr3. This white hygroscopic solid is of interest as a component of scintillation counters.
Lanthanum(III) bromide (LaBr3) is an inorganic halide salt of lanthanum. When pure, it is a colorless white powder. The single crystals of LaBr3 are hexagonal crystals with melting point of 783 °C. It is highly hygroscopic and water-soluble. There are several hydrates, La3Br·x H2O, of the salt also known. It is often used as a source of lanthanum in chemical synthesis and as a scintillation material in certain applications.
Lyso can refer to:
References
- http://authors.library.caltech.edu/8520/1/CHEieeetns07b.pdf
- http://omegapiezo.com/crystal_scintillators.html
| This inorganic compound–related article is a stub. You can help Wikipedia by expanding it. |