Related Research Articles

Gastrulation is the stage in the early embryonic development of most animals, during which the blastula, or in mammals the blastocyst is reorganized into a multilayered structure known as the gastrula. Before gastrulation, the embryo is a continuous epithelial sheet of cells; by the end of gastrulation, the embryo has begun differentiation to establish distinct cell lineages, set up the basic axes of the body, and internalized one or more cell types including the prospective gut.
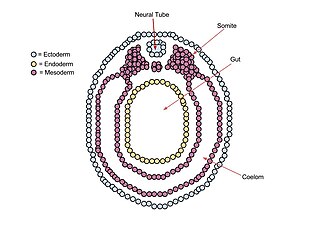
A neurula is a vertebrate embryo at the early stage of development in which neurulation occurs. The neurula stage is preceded by the gastrula stage; consequentially, neurulation is preceded by gastrulation. Neurulation marks the beginning of the process of organogenesis.
The primitive node is the organizer for gastrulation in most amniote embryos. In birds it is known as Hensen's node, and in amphibians it is known as the Spemann-Mangold organizer. It is induced by the Nieuwkoop center in amphibians, or by the posterior marginal zone in amniotes including birds.

The primitive streak is a structure that forms in the early embryo in amniotes. In amphibians the equivalent structure is the blastopore. During early embryonic development, the embryonic disc becomes oval shaped, and then pear-shaped with the broad end towards the anterior, and the narrower region projected to the posterior. The primitive streak forms a longitudinal midline structure in the narrower posterior (caudal) region of the developing embryo on its dorsal side. At first formation the primitive streak extends for half the length of the embryo. In the human embryo this appears by stage 6, about 17 days.

Bone morphogenetic protein 4 is a protein that in humans is encoded by BMP4 gene. BMP4 is found on chromosome 14q22-q23.
Chordin is a protein with a prominent role in dorsal–ventral patterning during early embryonic development. In humans it is encoded for by the CHRD gene.
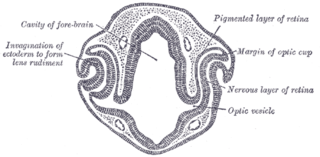
Eye formation in the human embryo begins at approximately three weeks into embryonic development and continues through the tenth week. Cells from both the mesodermal and the ectodermal tissues contribute to the formation of the eye. Specifically, the eye is derived from the neuroepithelium, surface ectoderm, and the extracellular mesenchyme which consists of both the neural crest and mesoderm.
In the field of developmental biology, regional differentiation is the process by which different areas are identified in the development of the early embryo. The process by which the cells become specified differs between organisms.

Cerberus is a protein that in humans is encoded by the CER1 gene. Cerberus is a signaling molecule which contributes to the formation of the head, heart and left-right asymmetry of internal organs. This gene varies slightly from species to species but its overall functions seem to be similar.

Homeobox protein Hox-A9 is a protein that in humans is encoded by the HOXA9 gene.

Homeobox protein Hox-A10 is a protein that in humans is encoded by the HOXA10 gene.

Homeobox protein Hox-B6 is a protein that in humans is encoded by the HOXB6 gene.

Homeobox Protein HB24 is a protein that in humans is encoded by the HLX gene.

Nodal homolog is a secretory protein that in humans is encoded by the NODAL gene which is located on chromosome 10q22.1. It belongs to the transforming growth factor beta (TGF-β) superfamily. Like many other members of this superfamily it is involved in cell differentiation in early embryogenesis, playing a key role in signal transfer from the primitive node, in the anterior primitive streak, to lateral plate mesoderm (LPM).

Homeobox protein goosecoid(GSC) is a homeobox protein that is encoded in humans by the GSC gene. Like other homeobox proteins, goosecoid functions as a transcription factor involved in morphogenesis. In Xenopus, GSC is thought to play a crucial role in the phenomenon of the Spemann-Mangold organizer. Through lineage tracing and timelapse microscopy, the effects of GSC on neighboring cell fates could be observed. In an experiment that injected cells with GSC and observed the effects of uninjected cells, GSC recruited neighboring uninjected cells in the dorsal blastopore lip of the Xenopus gastrula to form a twinned dorsal axis, suggesting that the goosecoid protein plays a role in the regulation and migration of cells during gastrulation.
The Nodal signaling pathway is a signal transduction pathway important in regional and cellular differentiation during embryonic development.

In Xenopus laevis, the specification of the three germ layers occurs at the blastula stage. Great efforts have been made to determine the factors that specify the endoderm and mesoderm. On the other hand, only a few examples of genes that are required for ectoderm specification have been described in the last decade. The first molecule identified to be required for the specification of ectoderm was the ubiquitin ligase Ectodermin ; later, it was found that the deubiquitinating enzyme, FAM/USP9x, is able to overcome the effects of ubiquitination made by Ectodermin in Smad4. Two transcription factors have been proposed to control gene expression of ectodermal specific genes: POU91/Oct3/4 and FoxIe1/Xema. A new factor specific for the ectoderm, XFDL156, has shown to be essential for suppression of mesoderm differentiation from pluripotent cells.
tinman, or tin is an Nk2-homeobox containing transcription factor first isolated in Drosophila flies. The human homolog is the Nkx2-5 gene. tinman is expressed in the precardiac mesoderm and is responsible for the differentiation, proliferation, and specification of cardiac progenitor cells. This gene is named after the character Tin Woodman who lacks a heart, as flies with nonfunctional tinman genes have cardiac deformities.
Lim-1 is a homeobox transcription factor. This transcription factor is found in adults in the cerebellum, kidneys, and cerebrum, but plays a larger role in development of the fetal head and the female reproductive tract during gestation. During development it is found in the anterior visceral endoderm, is in tissues formed by the primitive streak, and is required in both tissues for head formation. Lim1 is a member of the LIM homeobox gene and encodes a 406 amino acid protein.

Musashi-2, also known as Musashi RNA binding protein 2, is a protein that in humans is encoded by the MSI2 gene. Like its homologue musashi-1 (MSI1), it is an RNA-binding protein involved in stemness.
References
- ↑
- ↑
- 1 2
- ↑
- ↑
- Enforced expression of the homeobox gene Mixl1 impairs hematopoietic differentiation and results in acute myeloid leukemia. Glaser, S., Metcalf, D., Wu, L., Hart, A.H., Dirago, L., Mifsud, S., D'Amico, A., Dagger, S., Campo, C., Chan, A.C., Izon, D.J., Robb, L. Proc. Natl. Acad. Sci. U.S.A. (2006) PMID 17060613