Related Research Articles

An atom is the smallest unit of ordinary matter that forms a chemical element. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small, typically around 100 picometers across. They are so small that accurately predicting their behavior using classical physics—as if they were tennis balls, for example—is not possible due to quantum effects.

Xenon is a chemical element with the symbol Xe and atomic number 54. It is a colorless, dense, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the formation of xenon hexafluoroplatinate, the first noble gas compound to be synthesized.
Livermorium is a synthetic chemical element with the symbol Lv and has an atomic number of 116. It is an extremely radioactive element that has only been created in the laboratory and has not been observed in nature. The element is named after the Lawrence Livermore National Laboratory in the United States, which collaborated with the Joint Institute for Nuclear Research (JINR) in Dubna, Russia to discover livermorium during experiments conducted between 2000 and 2006. The name of the laboratory refers to the city of Livermore, California where it is located, which in turn was named after the rancher and landowner Robert Livermore. The name was adopted by IUPAC on May 30, 2012. Four isotopes of livermorium are known, with mass numbers between 290 and 293 inclusive; the longest-lived among them is livermorium-293 with a half-life of about 60 milliseconds. A fifth possible isotope with mass number 294 has been reported but not yet confirmed.
Moscovium is a synthetic element with the symbol Mc and atomic number 115. It was first synthesized in 2003 by a joint team of Russian and American scientists at the Joint Institute for Nuclear Research (JINR) in Dubna, Russia. In December 2015, it was recognized as one of four new elements by the Joint Working Party of international scientific bodies IUPAC and IUPAP. On 28 November 2016, it was officially named after the Moscow Oblast, in which the JINR is situated.
In physical organic chemistry, a kinetic isotope effect (KIE) is the change in the reaction rate of a chemical reaction when one of the atoms in the reactants is replaced by one of its isotopes. Formally, it is the ratio of rate constants for the reactions involving the light (kL) and the heavy (kH) isotopically substituted reactants (isotopologues):
Mass-independent isotope fractionation or Non-mass-dependent fractionation (NMD), refers to any chemical or physical process that acts to separate isotopes, where the amount of separation does not scale in proportion with the difference in the masses of the isotopes. Most isotopic fractionations are caused by the effects of the mass of an isotope on atomic or molecular velocities, diffusivities or bond strengths. Mass-independent fractionation processes are less common, occurring mainly in photochemical and spin-forbidden reactions. Observation of mass-independently fractionated materials can therefore be used to trace these types of reactions in nature and in laboratory experiments.

Singlet oxygen, systematically named dioxygen(singlet) and dioxidene, is a gaseous inorganic chemical with the formula O=O, which is in a quantum state where all electrons are spin paired. It is kinetically unstable at ambient temperature, but the rate of decay is slow.
CIDNP, often pronounced like "kidnip", is a nuclear magnetic resonance (NMR) technique that is used to study chemical reactions that involve radicals. It detects the non-Boltzmann (non-thermal) nuclear spin state distribution produced in these reactions as enhanced absorption or emission signals.
In organic chemistry, the term 2-norbornyl cation describes one of the three carbocations formed from derivatives of norbornane. Though 1-norbornyl and 7-norbornyl cations have been studied, the most extensive studies and vigorous debates have been centered on the exact structure of the 2-norbornyl cation.

Frank Henry Westheimer was an American chemist. He taught at the University of Chicago from 1936 to 1954, and at Harvard University from 1953 to 1983, becoming the Morris Loeb Professor of Chemistry in 1960, and Professor Emeritus in 1983. The Westheimer medal was established in his honor in 2002.
Spin chemistry is a sub-field of chemistry and physics, positioned at the intersection of chemical kinetics, photochemistry, magnetic resonance and free radical chemistry, that deals with magnetic and spin effects in chemical reactions. Spin chemistry concerns phenomena such as chemically induced dynamic nuclear polarization (CIDNP), chemically induced electron polarization (CIDEP), magnetic isotope effects in chemical reactions, and it is hypothesized to be key in the underlying mechanism for avian magnetoreception and consciousness.
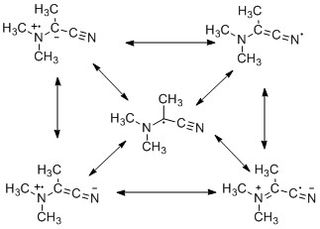
The captodative effect is the stabilization of radicals by a synergistic effect of an electron-withdrawing substituent and an electron-donating substituent. The name originates as the electron-withdrawing group (EWG) is sometimes called the "captor" group, whilst the electron-donating group (EDG) is the "dative" substituent. Olefins with this substituent pattern are sometime described as captodative. Radical reactions play an integral role in several chemical reactions and are also important to the field of polymer science.
Physical organic chemistry, a term coined by Louis Hammett in 1940, refers to a discipline of organic chemistry that focuses on the relationship between chemical structures and reactivity, in particular, applying experimental tools of physical chemistry to the study of organic molecules. Specific focal points of study include the rates of organic reactions, the relative chemical stabilities of the starting materials, reactive intermediates, transition states, and products of chemical reactions, and non-covalent aspects of solvation and molecular interactions that influence chemical reactivity. Such studies provide theoretical and practical frameworks to understand how changes in structure in solution or solid-state contexts impact reaction mechanism and rate for each organic reaction of interest.
Quantum biology is the study of applications of quantum mechanics and theoretical chemistry to aspects of biology that cannot be accurately described by the classical laws of physics. An understanding of fundamental quantum interactions is important because they determine the properties of the next level of organization in biological systems.

Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field and respond by producing an electromagnetic signal with a frequency characteristic of the magnetic field at the nucleus. This process occurs near resonance, when the oscillation frequency matches the intrinsic frequency of the nuclei, which depends on the strength of the static magnetic field, the chemical environment, and the magnetic properties of the isotope involved; in practical applications with static magnetic fields up to ca. 20 tesla, the frequency is similar to VHF and UHF television broadcasts (60–1000 MHz). NMR results from specific magnetic properties of certain atomic nuclei. Nuclear magnetic resonance spectroscopy is widely used to determine the structure of organic molecules in solution and study molecular physics and crystals as well as non-crystalline materials. NMR is also routinely used in advanced medical imaging techniques, such as in magnetic resonance imaging (MRI).
Nuclear magnetic resonance crystallography is a method which utilizes primarily NMR spectroscopy to determine the structure of solid materials on the atomic scale. Thus, solid-state NMR spectroscopy would be used primarily, possibly supplemented by quantum chemistry calculations, powder diffraction etc. If suitable crystals can be grown, any crystallographic method would generally be preferred to determine the crystal structure comprising in case of organic compounds the molecular structures and molecular packing. The main interest in NMR crystallography is in microcrystalline materials which are amenable to this method but not to X-ray, neutron and electron diffraction. This is largely because interactions of comparably short range are measured in NMR crystallography.
Sharon Hammes-Schiffer is a physical chemist who has contributed to theoretical and computational chemistry. She is currently a Sterling Professor of Chemistry at Yale University. She has served as senior editor and deputy editor of the Journal of Physical Chemistry and advisory editor for Theoretical Chemistry Accounts. As of 1 January 2015 she is editor-in-chief of Chemical Reviews.
Maria-Elisabeth Michel-Beyerle is a German chemist. From 1974 to 2000, she was a professor of Physical Chemistry at the Technical University of Munich. Among other awards, she has received the 2000 Bavarian Order of Merit, the highest service order bestowed by the Free State of Bavaria, for her work on photosynthesis.

Position-specific isotope analysis, also called site-specific isotope analysis, is a branch of isotope analysis aimed at determining the isotopic composition of a particular atom position in a molecule. Isotopes are elemental variants with different numbers of neutrons in their nuclei, thereby having different atomic masses. Isotopes are found in varying natural abundances depending on the element; their abundances in specific compounds can vary from random distributions due to environmental conditions that act on the mass variations differently. These differences in abundances are called "fractionations," which are characterized via stable isotope analysis.
Christiane Renate Timmel is a German chemist who is Director of the Centre for Advanced Electron Spin Resonance at the University of Oxford. Her group make use of electron-spin resonance to understand long-range structures in chemical and biological systems. Timmel was awarded the Tilden Prize on 2020 by the Royal Society of Chemistry for her contributions to electron-spin resonance.
References
- ↑ Turro, Nicholas J.; Kraeutler, Bernhard (1980). "Magnetic field and magnetic isotope effects in organic photochemical reactions. A novel probe of reaction mechanisms and a method for enrichment of magnetic isotopes". Accounts of Chemical Research. 13 (10): 369–377. doi:10.1021/ar50154a005.
- ↑ Steiner, Ulrich E. (1989). "Magnetic field effects in chemical kinetics and related phenomena". Chemical Reviews. 89: 51–147. doi:10.1021/cr00091a003.
- ↑ Yi, Qin Gao; Marcus, R.A. (2001). "Strange and Unconventional Isotope Effects in Ozone Formation". Science. 293 (5528): 259–263. Bibcode:2001Sci...293..259G. doi:10.1126/science.1058528. PMID 11387441. S2CID 867229.