
In polymer physics, Maltese Cross is a set of four symmetrically disposed sectors of high extinction that is displayed when a polymer is observed under polarized lights. This is usually observed when trying to observe spheruliltes in polymers.

In polymer physics, Maltese Cross is a set of four symmetrically disposed sectors of high extinction that is displayed when a polymer is observed under polarized lights. This is usually observed when trying to observe spheruliltes in polymers.

A polymer is a substance or material consisting of very large molecules, or macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals.

In chemistry, vinyl or ethenyl (abbreviated as Vi) is the functional group with the formula −CH=CH2. It is the ethylene (IUPAC ethene) molecule (H2C=CH2) with one fewer hydrogen atom. The name is also used for any compound containing that group, namely R−CH=CH2 where R is any other group of atoms.

In chemistry, a silicate is any member of a family of anions consisting of silicon and oxygen, usually with the general formula [SiO(4−2x)−
4−x]
n, where 0 ≤ x < 2. The family includes orthosilicate SiO4−
4, metasilicate SiO2−
3, and pyrosilicate Si
2O6−
7. The name is also used for any salt of such anions, such as sodium metasilicate; or any ester containing the corresponding chemical group, such as tetramethyl orthosilicate.

Differential scanning calorimetry (DSC) is a thermoanalytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference is measured as a function of temperature. Both the sample and reference are maintained at nearly the same temperature throughout the experiment. Generally, the temperature program for a DSC analysis is designed such that the sample holder temperature increases linearly as a function of time. The reference sample should have a well-defined heat capacity over the range of temperatures to be scanned.

Polypropylene (PP), also known as polypropene, is a thermoplastic polymer used in a wide variety of applications. It is produced via chain-growth polymerization from the monomer propylene.
In chemistry isomerization or isomerisation is the process in which a molecule, ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomerization. When the isomerization occurs intramolecularly it may be called a rearrangement reaction.
In materials science and continuum mechanics, viscoelasticity is the property of materials that exhibit both viscous and elastic characteristics when undergoing deformation. Viscous materials, like water, resist shear flow and strain linearly with time when a stress is applied. Elastic materials strain when stretched and immediately return to their original state once the stress is removed.

A copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained by copolymerization of two monomer species are sometimes called bipolymers. Those obtained from three and four monomers are called terpolymers and quaterpolymers, respectively.

Adhesion is the tendency of dissimilar particles or surfaces to cling to one another.

Crazing is the phenomenon that produces a network of fine cracks on the surface of a material, for example in a glaze layer. Crazing frequently precedes fracture in some glassy thermoplastic polymers. As it only takes place under tensile stress, the plane of the crazing corresponds to the stress direction. The effect is visibly distinguishable from other types of fine cracking because the crazing region has different refractive indices from surrounding material. Crazing occurs in regions of high hydrostatic tension, or in regions of very localized yielding, which leads to the formation of interpenetrating microvoids and small fibrils. If an applied tensile load is sufficient, these bridges elongate and break, causing the microvoids to grow and coalesce; as microvoids coalesce, cracks begin to form.

Linear low-density polyethylene (LLDPE) is a substantially linear polymer (polyethylene), with significant numbers of short branches, commonly made by copolymerization of ethylene with longer-chain olefins. Linear low-density polyethylene differs structurally from conventional low-density polyethylene (LDPE) because of the absence of long chain branching. The linearity of LLDPE results from the different manufacturing processes of LLDPE and LDPE. In general, LLDPE is produced at lower temperatures and pressures by copolymerization of ethylene and such higher alpha-olefins as butene, hexene, or octene. The copolymerization process produces an LLDPE polymer that has a narrower molecular weight distribution than conventional LDPE and in combination with the linear structure, significantly different rheological properties.

Flory–Huggins solution theory is a lattice model of the thermodynamics of polymer solutions which takes account of the great dissimilarity in molecular sizes in adapting the usual expression for the entropy of mixing. The result is an equation for the Gibbs free energy change for mixing a polymer with a solvent. Although it makes simplifying assumptions, it generates useful results for interpreting experiments.
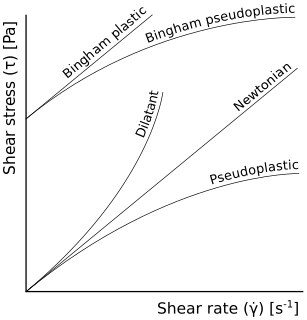
In rheology, shear thinning is the non-Newtonian behavior of fluids whose viscosity decreases under shear strain. It is sometimes considered synonymous for pseudoplastic behaviour, and is usually defined as excluding time-dependent effects, such as thixotropy.
A polymer blend, or polymer mixture, is a member of a class of materials analogous to metal alloys, in which at least two polymers are blended together to create a new material with different physical properties.
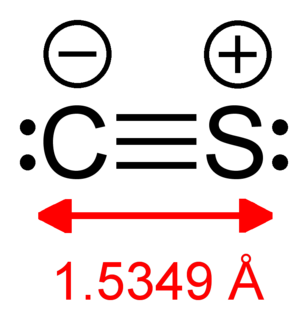
Carbon monosulfide is a chemical compound with the formula CS. This diatomic molecule is the sulfur analogue of carbon monoxide, and is unstable as a solid or a liquid, but it has been observed as a gas both in the laboratory and in the interstellar medium. The molecule resembles carbon monoxide with a triple bond between carbon and sulfur. The molecule is not intrinsically unstable, but it tends to polymerize. This tendency reflects the greater stability of C–S single bonds.

Reptation is the thermal motion of very long linear, entangled macromolecules in polymer melts or concentrated polymer solutions. Derived from the word reptile, reptation suggests the movement of entangled polymer chains as being analogous to snakes slithering through one another. Pierre-Gilles de Gennes introduced the concept of reptation into polymer physics in 1971 to explain the dependence of the mobility of a macromolecule on its length. Reptation is used as a mechanism to explain viscous flow in an amorphous polymer. Sir Sam Edwards and Masao Doi later refined reptation theory. Similar phenomena also occur in proteins.

Temperature-responsive polymers or thermoresponsive polymers are polymers that exhibit a drastic and discontinuous change of their physical properties with temperature. The term is commonly used when the property concerned is solubility in a given solvent, but it may also be used when other properties are affected. Thermoresponsive polymers belong to the class of stimuli-responsive materials, in contrast to temperature-sensitive materials, which change their properties continuously with environmental conditions. In a stricter sense, thermoresponsive polymers display a miscibility gap in their temperature-composition diagram. Depending on whether the miscibility gap is found at high or low temperatures, an upper or lower critical solution temperature exists, respectively.
ParM is a prokaryotic actin homologue which provides the force to drive copies of the R1 plasmid to opposite ends of rod shaped bacteria before cytokinesis.
Crystallization of polymers is a process associated with partial alignment of their molecular chains. These chains fold together and form ordered regions called lamellae, which compose larger spheroidal structures named spherulites. Polymers can crystallize upon cooling from melting, mechanical stretching or solvent evaporation. Crystallization affects optical, mechanical, thermal and chemical properties of the polymer. The degree of crystallinity is estimated by different analytical methods and it typically ranges between 10 and 80%, with crystallized polymers often called "semi-crystalline". The properties of semi-crystalline polymers are determined not only by the degree of crystallinity, but also by the size and orientation of the molecular chains.
Recoil is a rheological phenomenon observed only in non-Newtonian fluids that is characterized by a moving fluid's ability to snap back to a previous position when external forces are removed. Recoil is a result of the fluid's elasticity and memory where the speed and acceleration by which the fluid moves depends on the molecular structure and the location to which it returns depends on the conformational entropy. This effect is observed in numerous non-Newtonian liquids to a small degree, but is prominent in some materials such as molten polymers.