
Labcorp Drug Development is a contract research organization (CRO) headquartered in Burlington, North Carolina, providing nonclinical, preclinical, clinical and commercialization services to pharmaceutical and biotechnology industries. Formerly called Covance, the company is part of Labcorp, which employs more than 70,000 people worldwide. Labcorp Drug Development claims to provide the world's largest central laboratory network. Laboratory Corporation of America Holdings (Labcorp), was rated one of the best places to work for LGBTQ equality by the Human Rights Campaign in 2018-2021. Labcorp Drug Development has been subject to harassment from animal rights groups for its animal testing procedures.
Antiserum is human or nonhuman blood serum containing monoclonal or polyclonal antibodies that is used to spread passive immunity to many diseases via blood donation (plasmapheresis). For example, convalescent serum, passive antibody transfusion from a previous human survivor, used to be the only known effective treatment for ebola infection with a high success rate of 7 out of 8 patients surviving.
Pharming, a portmanteau of "farming" and "pharmaceutical", refers to the use of genetic engineering to insert genes that code for useful pharmaceuticals into host animals or plants that would otherwise not express those genes, thus creating a genetically modified organism (GMO). Pharming is also known as molecular farming, molecular pharming or biopharming.
A biopharmaceutical, also known as a biologic(al) medical product, or biologic, is any pharmaceutical drug product manufactured in, extracted from, or semisynthesized from biological sources. Different from totally synthesized pharmaceuticals, they include vaccines, whole blood, blood components, allergenics, somatic cells, gene therapies, tissues, recombinant therapeutic protein, and living medicines used in cell therapy. Biologics can be composed of sugars, proteins, nucleic acids, or complex combinations of these substances, or may be living cells or tissues. They are isolated from living sources—human, animal, plant, fungal, or microbial. They can be used in both human and animal medicine.

Nicotiana benthamiana is a close relative of tobacco and species of Nicotiana indigenous to Australia.
Charles Joel Arntzen is a plant molecular biologist.

Ebola, also known as Ebola virus disease (EVD) and Ebola hemorrhagic fever (EHF), is a viral hemorrhagic fever in humans and other primates, caused by ebolaviruses. Symptoms typically start anywhere between two days and three weeks after becoming infected with the virus. The first symptoms are usually fever, sore throat, muscle pain, and headaches. These are usually followed by vomiting, diarrhoea, rash and decreased liver and kidney function, at which point, some people begin to bleed both internally and externally. The disease kills between 25% and 90% of those infected—about 50% on average. Death is often due to shock from fluid loss, and typically occurs between six and 16 days after the first symptoms appear.

ZMapp is an experimental biopharmaceutical drug comprising three chimeric monoclonal antibodies under development as a treatment for Ebola virus disease. Two of the three components were originally developed at the Public Health Agency of Canada's National Microbiology Laboratory (NML), and the third at the U.S. Army Medical Research Institute of Infectious Diseases; the cocktail was optimized by Gary Kobinger, a research scientist at the NML and underwent further development under license by Mapp Biopharmaceutical. ZMapp was first used on humans during the 2014 West Africa Ebola virus outbreak, having only been previously tested on animals and not yet subjected to a randomized controlled trial. The NIH ran a clinical trial starting in January 2015 with subjects from Sierra Leone, Guinea, and Liberia aiming to enroll 200 people, but the epidemic waned and the trial closed early, leaving it too statistically underpowered to give a meaningful result about whether ZMapp worked.
TKM-Ebola was an experimental antiviral drug for Ebola disease that was developed by Arbutus Biopharma in Vancouver, Canada. The drug candidate was formerly known as Ebola-SNALP.
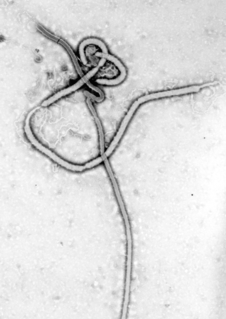
Recombinant vesicular stomatitis virus–Zaire Ebola virus (rVSV-ZEBOV), also known as Ebola Zaire vaccine live and sold under the brand name Ervebo, is an Ebola vaccine for adults that prevents Ebola caused by the Zaire ebolavirus. When used in ring vaccination, rVSV-EBOV has shown a high level of protection. Around half the people given the vaccine have mild to moderate adverse effects that include headache, fatigue, and muscle pain.

Four laboratory-confirmed cases of Ebola virus disease occurred in the United States in 2014. Eleven cases were reported, including these four cases and seven cases medically evacuated from other countries. The first was reported in September 2014. Nine of the people contracted the disease outside the US and traveled into the country, either as regular airline passengers or as medical evacuees; of those nine, two died. Two people contracted Ebola in the United States. Both were nurses who treated an Ebola patient; both recovered.

Ebola vaccines are vaccines either approved or in development to prevent Ebola. The first vaccine to be approved in the United States was rVSV-ZEBOV in December 2019. It had been used extensively in the Kivu Ebola epidemic under a compassionate use protocol. During the early 21st century, several vaccine candidates displayed efficacy to protect nonhuman primates against lethal infection.

Galidesivir is an antiviral drug, an adenosine analog. It is developed by BioCryst Pharmaceuticals with funding from NIAID, originally intended as a treatment for hepatitis C, but subsequently developed as a potential treatment for deadly filovirus infections such as Ebola virus disease, Marburg virus disease, and Zika virus. Currently, galidesivir is under phase 1 human trial in Brazil for coronavirus.

In 2014, Ebola virus disease in Spain occurred due to two patients with cases of the disease contracted during the Ebola virus epidemic in West Africa; they were medically evacuated. A failure in infection control in the treatment of the second patient led to an isolated infection of Ebola virus disease in a health worker in Spain itself. The health worker survived her Ebola infection, and has since been declared infection-free.

cAd3-ZEBOV is an experimental vaccine for two ebolaviruses, Ebola virus and Sudan virus, developed by scientists at GlaxoSmithKline (GSK) and tested by National Institute of Allergy and Infectious Disease (NIAID). This vaccine is derived from a chimpanzee adenovirus, Chimp Adenovirus type 3 (ChAd3), genetically engineered to express glycoproteins from the Zaire and Sudan species of ebolavirus to provoke an immune response against them. Simultaneous phase 1 trials of this vaccine commenced in September 2014, being administered to volunteers in Oxford and Bethesda. During October the vaccine is being administered to a further group of volunteers in Mali. If this phase is completed successfully, the vaccine will be fast tracked for use in the Ebola virus epidemic in West Africa. In preparation for this, GSK is preparing a stockpile of 10,000 doses.

There is no cure or specific treatment for the Ebola virus disease that is currently approved for market, although various experimental treatments are being developed. For past and current Ebola epidemics, treatment has been primarily supportive in nature.
Ansuvimab, sold under the brand name Ebanga, is a monoclonal antibody medication for the treatment of Zaire ebolavirus (Ebolavirus) infection.

Jean-Jacques Muyembe is a Congolese microbiologist. He is the general director of the Democratic Republic of the Congo Institut National pour la Recherche Biomedicale (INRB). He was part of team at the Yambuku Catholic Mission Hospital that investigated the first Ebola outbreak, and was part of the effort that discovered Ebola as a new disease, although his exact role is still subject to controversy. In 2016, he led the research that designed, along with other researchers at the INRB and the National Institute of Health Vaccine Research Center in the US, one of the most promising treatment for Ebola, mAb114. The treatment was successfully experimented during recent outbreaks in the DRC, on the express decision of the then DRC Minister of Health, Dr Oly Ilunga, despite a prior negative advice from the World Health Organization.
Atoltivimab/maftivimab/odesivimab, developed as REGN-EB3 and sold under the brand name Inmazeb, is a fixed-dose combination of three monoclonal antibodies for the treatment of Zaire ebolavirus. It contains atoltivimab, maftivimab, and odesivimab-ebgn and was developed by Regeneron Pharmaceuticals.
Xiangguo Qiu is a Chinese Canadian virologist, former adjunct professor of Medical microbiology at the University of Manitoba and former head of the Vaccine Development and Antiviral Therapies section in the Special Pathogen Program of the Public Health Agency of Canada (PHAC).










