Related Research Articles

G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. They are coupled with G proteins. They pass through the cell membrane seven times in the form of six loops of amino acid residues, which is why they are sometimes referred to as seven-transmembrane receptors. Ligands can bind either to the extracellular N-terminus and loops or to the binding site within transmembrane helices. They are all activated by agonists, although a spontaneous auto-activation of an empty receptor has also been observed.

The adrenergic receptors or adrenoceptors are a class of G protein-coupled receptors that are targets of many catecholamines like norepinephrine (noradrenaline) and epinephrine (adrenaline) produced by the body, but also many medications like beta blockers, beta-2 (β2) agonists and alpha-2 (α2) agonists, which are used to treat high blood pressure and asthma, for example.

Penbutolol is a medication in the class of beta blockers, used in the treatment of high blood pressure. Penbutolol is able to bind to both beta-1 adrenergic receptors and beta-2 adrenergic receptors, thus making it a non-selective β blocker. Penbutolol is a sympathomimetic drug with properties allowing it to act as a partial agonist at β adrenergic receptors.

The beta-1 adrenergic receptor, also known as ADRB1, can refer to either the protein-encoding gene or one of the four adrenergic receptors. It is a G-protein coupled receptor associated with the Gs heterotrimeric G-protein that is expressed predominantly in cardiac tissue. In addition to cardiac tissue, beta-1 adrenergic receptors are also expressed in the cerebral cortex.
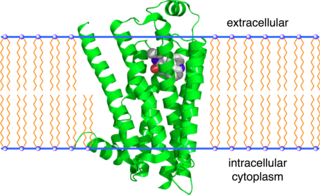
The beta-2 adrenergic receptor, also known as ADRB2, is a cell membrane-spanning beta-adrenergic receptor that binds epinephrine (adrenaline), a hormone and neurotransmitter whose signaling, via adenylate cyclase stimulation through trimeric Gs proteins, increases cAMP, and, via downstream L-type calcium channel interaction, mediates physiologic responses such as smooth muscle relaxation and bronchodilation.

Arrestins are a small family of proteins important for regulating signal transduction at G protein-coupled receptors. Arrestins were first discovered as a part of a conserved two-step mechanism for regulating the activity of G protein-coupled receptors (GPCRs) in the visual rhodopsin system by Hermann Kühn, Scott Hall, and Ursula Wilden and in the β-adrenergic system by Martin J. Lohse and co-workers.

G-protein-coupled receptor kinase 2 (GRK2) is an enzyme that in humans is encoded by the ADRBK1 gene. GRK2 was initially called Beta-adrenergic receptor kinase, and is a member of the G protein-coupled receptor kinase subfamily of the Ser/Thr protein kinases that is most highly similar to GRK3(βARK2).
Gq protein alpha subunit is a family of heterotrimeric G protein alpha subunits. This family is also commonly called the Gq/11 (Gq/G11) family or Gq/11/14/15 family to include closely related family members. G alpha subunits may be referred to as Gq alpha, Gαq, or Gqα. Gq proteins couple to G protein-coupled receptors to activate beta-type phospholipase C (PLC-β) enzymes. PLC-β in turn hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) to diacyl glycerol (DAG) and inositol trisphosphate (IP3). IP3 acts as a second messenger to release stored calcium into the cytoplasm, while DAG acts as a second messenger that activates protein kinase C (PKC).

Protein kinase C epsilon type (PKCε) is an enzyme that in humans is encoded by the PRKCE gene. PKCε is an isoform of the large PKC family of protein kinases that play many roles in different tissues. In cardiac muscle cells, PKCε regulates muscle contraction through its actions at sarcomeric proteins, and PKCε modulates cardiac cell metabolism through its actions at mitochondria. PKCε is clinically significant in that it is a central player in cardioprotection against ischemic injury and in the development of cardiac hypertrophy.

Regulator of G-protein signaling 2 is a protein that in humans is encoded by the RGS2 gene. It is part of a larger family of RGS proteins that control signalling through G-protein coupled receptors (GPCR).

Beta-arrestin-2, also known as arrestin beta-2, is an intracellular protein that in humans is encoded by the ARRB2 gene.

Arrestin, beta 1, also known as ARRB1, is a protein which in humans is encoded by the ARRB1 gene.

G protein-coupled receptor kinase 5 is a member of the G protein-coupled receptor kinase subfamily of the Ser/Thr protein kinases, and is most highly similar to GRK4 and GRK6. The protein phosphorylates the activated forms of G protein-coupled receptors to regulate their signaling.
In the field of molecular biology, the cAMP-dependent pathway, also known as the adenylyl cyclase pathway, is a G protein-coupled receptor-triggered signaling cascade used in cell communication.
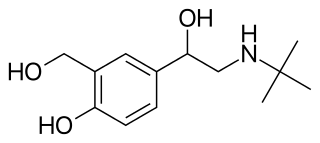
Beta adrenergic agonists or beta agonists are medications that relax muscles of the airways, causing widening of the airways and resulting in easier breathing. They are a class of sympathomimetic agents, each acting upon the beta adrenoceptors. In general, pure beta-adrenergic agonists have the opposite function of beta blockers: beta-adrenoreceptor agonist ligands mimic the actions of both epinephrine- and norepinephrine- signaling, in the heart and lungs, and in smooth muscle tissue; epinephrine expresses the higher affinity. The activation of β1, β2 and β3 activates the enzyme, adenylate cyclase. This, in turn, leads to the activation of the secondary messenger cyclic adenosine monophosphate (cAMP); cAMP then activates protein kinase A (PKA) which phosphorylates target proteins, ultimately inducing smooth muscle relaxation and contraction of the cardiac tissue.

The G beta-gamma complex (Gβγ) is a tightly bound dimeric protein complex, composed of one Gβ and one Gγ subunit, and is a component of heterotrimeric G proteins. Heterotrimeric G proteins, also called guanosine nucleotide-binding proteins, consist of three subunits, called alpha, beta, and gamma subunits, or Gα, Gβ, and Gγ. When a G protein-coupled receptor (GPCR) is activated, Gα dissociates from Gβγ, allowing both subunits to perform their respective downstream signaling effects. One of the major functions of Gβγ is the inhibition of the Gα subunit.
Heterologous desensitization is the term for the unresponsiveness of cells to one or more agonists to which they are normally responsive. Typically, desensitization is a receptor-based phenomenon in which one receptor type, when bound to its ligand, becomes unable to further influence the signalling pathways by which it regulates cells and, in the case of cell surface membrane receptors, may thereafter be internalized. The desensitized receptor is degraded or freed of its activating ligand and re-cycled to a state where it is again able to respond to cognate ligands by activating its signalling pathways.

G-protein-coupled receptor kinase 3 (GRK3) is an enzyme that in humans is encoded by the ADRBK2 gene. GRK3 was initially called Beta-adrenergic receptor kinase 2 (βARK-2), and is a member of the G protein-coupled receptor kinase subfamily of the Ser/Thr protein kinases that is most highly similar to GRK2.
Beta adrenergic receptor kinase carboxyl-terminus is a peptide composed of the last 194 amino acid residues of the carboxyl-terminus of beta adrenergic receptor kinase 1 (βARK1). It binds the βγ subunits of G proteins located in the plasma membrane of cells. It is currently an experimental gene therapy for the treatment of heart failure.
The Raf kinase inhibitor protein (RKIP) is a kinase inhibitor protein, that regulates many signaling pathways within the cell. RKIP is a member of the phosphatidylethanolamine-binding protein family and has displayed disruptive regulation on the Raf-1-MEK1/2, ERK1/2 and NF-kappaB signalling pathways, by interaction with the Raf-1 kinase.
References
- ↑ "Martin Lohse wird Chef des Max-Delbrück-Centrums". Gesundheitsstadt Berlin (in German). 4 March 2016. Retrieved 27 November 2021.
- ↑ "Wechsel im Vorstand des MDC". MDC Berlin (in German). Retrieved 27 November 2021.
- ↑ Lohse, Martin J.; Benovic, Jeffrey L.; Codina, Juan; Caron, Marc G.; Lefkowitz, Robert J. (22 June 1990). "β-Arrestin: a Protein that Regulates β-adrenergic Receptor Function". Science. 248 (4962). American Association for the Advancement of Science (AAAS): 1547–1550. Bibcode:1990Sci...248.1547L. doi:10.1126/science.2163110. ISSN 0036-8075. PMID 2163110.
- ↑ Ungerer, M; Böhm, M; Elce, J S; Erdmann, E; Lohse, M J (1993). "Altered expression of beta-adrenergic receptor kinase and beta 1-adrenergic receptors in the failing human heart". Circulation. 87 (2). Ovid Technologies (Wolters Kluwer Health): 454–463. doi: 10.1161/01.cir.87.2.454 . ISSN 0009-7322. PMID 8381058.
- ↑ Engelhardt, S.; Hein, L.; Wiesmann, F.; Lohse, M. J. (8 June 1999). "Progressive hypertrophy and heart failure in 1-adrenergic receptor transgenic mice". Proceedings of the National Academy of Sciences. 96 (12): 7059–7064. doi: 10.1073/pnas.96.12.7059 . ISSN 0027-8424. PMC 22055 . PMID 10359838.
- ↑ Lorenz, Kristina; Schmitt, Joachim P; Schmitteckert, Eva M; Lohse, Martin J (7 December 2008). "A new type of ERK1/2 autophosphorylation causes cardiac hypertrophy". Nature Medicine. 15 (1). Springer Science and Business Media LLC: 75–83. doi:10.1038/nm.1893. ISSN 1078-8956. PMID 19060905. S2CID 13973823.
- ↑ Schmid, Evelyn; Neef, Stefan; Berlin, Christopher; Tomasovic, Angela; Kahlert, Katrin; Nordbeck, Peter; Deiss, Katharina; Denzinger, Sabrina; Herrmann, Sebastian; Wettwer, Erich; Weidendorfer, Markus; Becker, Daniel; Schäfer, Florian; Wagner, Nicole; Ergün, Süleyman; Schmitt, Joachim P; Katus, Hugo A; Weidemann, Frank; Ravens, Ursula; Maack, Christoph; Hein, Lutz; Ertl, Georg; Müller, Oliver J; Maier, Lars S; Lohse, Martin J; Lorenz, Kristina (19 October 2015). "Cardiac RKIP induces a beneficial β-adrenoceptor–dependent positive inotropy". Nature Medicine. 21 (11). Springer Science and Business Media LLC: 1298–1306. doi:10.1038/nm.3972. ISSN 1078-8956. PMID 26479924. S2CID 11418537.
- ↑ Vilardaga, Jean-Pierre; Bünemann, Moritz; Krasel, Cornelius; Castro, Mariàn; Lohse, Martin J (15 June 2003). "Measurement of the millisecond activation switch of G protein–coupled receptors in living cells". Nature Biotechnology. 21 (7). Springer Science and Business Media LLC: 807–812. doi:10.1038/nbt838. ISSN 1087-0156. PMID 12808462. S2CID 19520338.
- ↑ Hoffmann, Carsten; Gaietta, Guido; Bünemann, Moritz; Adams, Stephen R; Oberdorff-Maass, Silke; Behr, Björn; Vilardaga, Jean-Pierre; Tsien, Roger Y; Ellisman, Mark H; Lohse, Martin J (17 February 2005). "A FlAsH-based FRET approach to determine G protein–coupled receptor activation in living cells". Nature Methods. 2 (3). Springer Science and Business Media LLC: 171–176. doi:10.1038/nmeth742. ISSN 1548-7091. PMID 15782185. S2CID 6405686.
- ↑ Lohse, Martin J.; Nuber, Susanne; Hoffmann, Carsten (8 March 2012). Christopoulos, Arthur (ed.). "Fluorescence/Bioluminescence Resonance Energy Transfer Techniques to Study G-Protein-Coupled Receptor Activation and Signaling". Pharmacological Reviews. 64 (2). American Society for Pharmacology & Experimental Therapeutics (ASPET): 299–336. doi:10.1124/pr.110.004309. ISSN 0031-6997. PMID 22407612. S2CID 2042851.
- ↑ A. Bock, P. Annibale, C. Konrad, A. Hannawacker, S.E. Anton, I. Maiellaro, U. Zabel, S. Sivaramakrishnan, M. Falcke, M.J. Lohse: "Optical mapping of cAMP signaling at the nanometer scale." In: Cell. 182, 2020, pp. 1519-1530.
- ↑ S.E. Anton, C. Kayser, I. Maiellaro, K. Nemec, J. Möller, A. Koschinski, M. Zaccolo, P. Annibale, M. Falcke, M.J. Lohse, A. Bock: "Receptor-associated independent cAMP nanodomains mediate spatiotemporal specificity of GPCR signaling." In: Cell. 185, 2022, pp. 1130-1142.
- ↑ "Martin J. Lohse re-elected as Vice-President of the Leopoldina". Nationale Akademie der Wissenschaften Leopoldina. 6 October 2021. Retrieved 27 November 2021.
- ↑ "Curriculum Vitae Prof. Dr. Martin J. Lohse" (PDF). www.leopoldina.org. Retrieved April 23, 2024.
- ↑ Martin Lohse (Ed.): 'When the spark is lit. 200 years of the Society of German Natural Scientists and Physicians.' (in German) Passage-Verlag, Leipzig 2022, ISBN 978-3-95415-130-1