Related Research Articles

Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at 114 °C (237 °F), and boils to a violet gas at 184 °C (363 °F). The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek Ιώδης 'violet-coloured'.
Benedict's reagent is a chemical reagent and complex mixture of sodium carbonate, sodium citrate, and copper(II) sulfate pentahydrate. It is often used in place of Fehling's solution to detect the presence of reducing sugars. The presence of other reducing substances also gives a positive result. Such tests that use this reagent are called the Benedict's tests. A positive test with Benedict's reagent is shown by a color change from clear blue to brick-red with a precipitate.

In an aqueous solution, precipitation is the process of transforming a dissolved substance into an insoluble solid from a supersaturated solution. The solid formed is called the precipitate. In case of an inorganic chemical reaction leading to precipitation, the chemical reagent causing the solid to form is called the precipitant.
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency affects two billion people and is the leading preventable cause of intellectual disability.

Potassium tetraiodomercurate(II) is an inorganic compound with the chemical formula K2[HgI4]. It consists of potassium cations and tetraiodomercurate(II) anions. It is the active agent in Nessler's reagent, used for detection of ammonia.

Lead(II) iodide is a chemical compound with the formula PbI
2. At room temperature, it is a bright yellow odorless crystalline solid, that becomes orange and red when heated. It was formerly called plumbous iodide.
Classical qualitative inorganic analysis is a method of analytical chemistry which seeks to find the elemental composition of inorganic compounds. It is mainly focused on detecting ions in an aqueous solution, therefore materials in other forms may need to be brought to this state before using standard methods. The solution is then treated with various reagents to test for reactions characteristic of certain ions, which may cause color change, precipitation and other visible changes.

Tollens' reagent is a chemical reagent used to distinguish between aldehydes and ketones along with some alpha-hydroxy ketones which can tautomerize into aldehydes. The reagent consists of a solution of silver nitrate, ammonium hydroxide and some sodium hydroxide. It was named after its discoverer, the German chemist Bernhard Tollens. A positive test with Tollens' reagent is indicated by the precipitation of elemental silver, often producing a characteristic "silver mirror" on the inner surface of the reaction vessel.

Lugol's iodine, also known as aqueous iodine and strong iodine solution, is a solution of potassium iodide with iodine in water. It is a medication and disinfectant used for a number of purposes. Taken by mouth it is used to treat thyrotoxicosis until surgery can be carried out, protect the thyroid gland from radioactive iodine, and to treat iodine deficiency. When applied to the cervix it is used to help in screening for cervical cancer. As a disinfectant it may be applied to small wounds such as a needle stick injury. A small amount may also be used for emergency disinfection of drinking water.

The iodine–starch test is a chemical reaction that is used to test for the presence of starch or for iodine. The combination of starch and iodine is intensely blue-black. The interaction between starch and the triiodide anion is the basis for iodometry.
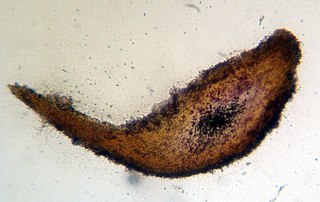
Melzer's reagent is a chemical reagent used by mycologists to assist with the identification of fungi, and by phytopathologists for fungi that are plant pathogens.

Ammonium metavanadate is the inorganic compound with the formula NH4VO3. It is a white salt, although samples are often yellow owing to impurities of V2O5. It is an important intermediate in the purification of vanadium.

Mercury(II) iodide is a chemical compound with the molecular formula HgI2. It is typically produced synthetically but can also be found in nature as the extremely rare mineral coccinite. Unlike the related mercury(II) chloride it is hardly soluble in water (<100 ppm).
In mycology a tissue or feature is said to be amyloid if it has a positive amyloid reaction when subjected to a crude chemical test using iodine as an ingredient of either Melzer's reagent or Lugol's solution, producing a blue to blue-black staining. The term "amyloid" is derived from the Latin amyloideus ("starch-like"). It refers to the fact that starch gives a similar reaction, also called an amyloid reaction. The test can be on microscopic features, such as spore walls or hyphal walls, or the apical apparatus or entire ascus wall of an ascus, or be a macroscopic reaction on tissue where a drop of the reagent is applied. Negative reactions, called inamyloid or nonamyloid, are for structures that remain pale yellow-brown or clear. A reaction producing a deep reddish to reddish-brown staining is either termed a dextrinoid reaction or a hemiamyloid reaction.
Ehrlich's reagent or Ehrlich reagent is a reagent containing p-dimethylaminobenzaldehyde (DMAB) and thus can act as an indicator to presumptively identify indoles and urobilinogen. Several Ehrlich tests use the reagent in a medical test; some are drug tests and others contribute to diagnosis of various diseases or adverse drug reactions. It is named after Nobel Prize winner Paul Ehrlich who used it to distinguish typhoid from simple diarrhoea.
Simon's reagent is used as a simple spot-test to presumptively identify alkaloids as well as other compounds. It reacts with secondary amines like MDMA and methamphetamine to give a blue solution.
The Froehde reagent is used as a simple spot-test to presumptively identify alkaloids, especially opioids, as well as other compounds. It is composed of a mixture of molybdic acid or a molybdate salt dissolved in hot, concentrated sulfuric acid, which is then dripped onto the substance being tested.
Iron(II) iodide is an inorganic compound with the chemical formula FeI2. It is used as a catalyst in organic reactions.

Dragendorff's reagent is a color reagent to detect alkaloids in a test sample or as a stain for chromatography plates. Alkaloids, if present in the solution of sample, will react with Dragendorff's reagent and produce an orange or orange-red precipitate. This reagent was invented by the German pharmacologist, Johann Georg Dragendorff (1836–1898) at the University of Dorpat.

Copper forms a rich variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. Copper compounds, whether organic complexes or organometallics, promote or catalyse numerous chemical and biological processes.
References
- ↑ "Mayer′s reagent". Sigma Aldrich. Retrieved 2012-02-08.
K2HgI4
- ↑ "Test Solutions". US Pharmacopeia. Retrieved 2012-02-08.