Related Research Articles
The National Institute of Standards and Technology (NIST) is an agency of the United States Department of Commerce whose mission is to promote American innovation and industrial competitiveness. NIST's activities are organized into physical science laboratory programs that include nanoscale science and technology, engineering, information technology, neutron research, material measurement, and physical measurement. From 1901 to 1988, the agency was named the National Bureau of Standards.

Cathodic protection is a technique used to control the corrosion of a metal surface by making it the cathode of an electrochemical cell. A simple method of protection connects the metal to be protected to a more easily corroded "sacrificial metal" to act as the anode. The sacrificial metal then corrodes instead of the protected metal. For structures such as long pipelines, where passive galvanic cathodic protection is not adequate, an external DC electrical power source is used to provide sufficient current.

James Sacra Albus was an American engineer, Senior NIST Fellow and founder and former chief of the Intelligent Systems Division of the Manufacturing Engineering Laboratory at the National Institute of Standards and Technology (NIST).

Jacob Rabinow was an engineer and inventor. He earned a total of 229 U.S. patents on a variety of mechanical, optical and electrical devices.
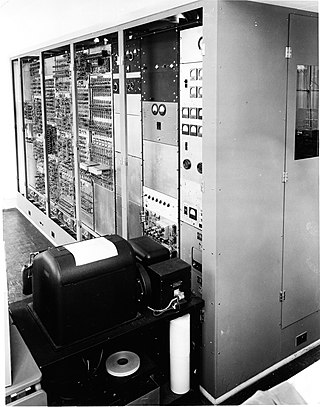
SEAC was a first-generation electronic computer, built in 1950 by the U.S. National Bureau of Standards (NBS) and was initially called the National Bureau of Standards Interim Computer, because it was a small-scale computer designed to be built quickly and put into operation while the NBS waited for more powerful computers to be completed. The team that developed SEAC was organized by Samuel N. Alexander. SEAC was demonstrated in April 1950 and was dedicated in June 1950; it is claimed to be the first fully operational stored-program electronic computer in the US.
Marcel Pourbaix was a Belgian chemist and pianist. He performed his most well known research at the University of Brussels, studying corrosion. His biggest achievement is the derivation of potential-pH, better known as “Pourbaix Diagrams”. Pourbaix Diagrams are thermodynamic charts constructed using the Nernst equation and visualize the relationship between possible phases of a system, bounded by lines representing the reactions that transport between them. They can be read much like a phase diagram.

Lyman James Briggs was an American engineer, physicist and administrator. He was a director of the National Bureau of Standards during the Great Depression and chairman of the Uranium Committee before America entered the Second World War. The Lyman Briggs College at Michigan State University is named in his honor.

Edgar Buckingham was an American physicist.
Michael A. Streicher was an American metallurgist and engineer who became internationally recognized for his work on the testing and development of corrosion-resistant stainless steel alloys. He published widely in technical journals and textbooks and received numerous patents for his inventions.
Corrosion engineering is an engineering specialty that applies scientific, technical, engineering skills, and knowledge of natural laws and physical resources to design and implement materials, structures, devices, systems, and procedures to manage corrosion. From a holistic perspective, corrosion is the phenomenon of metals returning to the state they are found in nature. The driving force that causes metals to corrode is a consequence of their temporary existence in metallic form. To produce metals starting from naturally occurring minerals and ores, it is necessary to provide a certain amount of energy, e.g. Iron ore in a blast furnace. It is therefore thermodynamically inevitable that these metals when exposed to various environments would revert to their state found in nature. Corrosion and corrosion engineering thus involves a study of chemical kinetics, thermodynamics, electrochemistry and materials science.

Ductile iron pipe is pipe made of ductile cast iron commonly used for potable water transmission and distribution. This type of pipe is a direct development of earlier cast iron pipe, which it has superseded.

Screw piles, sometimes referred to as screw-piles, screw piers, screw anchors, screw foundations, ground screws, helical piles, helical piers, or helical anchors are a steel screw-in piling and ground anchoring system used for building deep foundations. Screw piles are typically manufactured from high-strength steel using varying sizes of tubular hollow sections for the pile or anchors shaft.
A sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a primary metal from corrosion or rusting. It may also be used for galvanization.
DCVG is a survey technique used for assessing the effectiveness of corrosion protection on buried steel structures. In particular, oil and natural gas pipelines are routinely monitored using this technique to help locate coating faults and highlight deficiencies in their cathodic protection (CP) strategies.
Herbert Henry Uhlig was an American physical chemist who studied corrosion.

William Clyde Martin Jr. was an American physicist. After receiving his Ph.D. degree from Princeton University in 1956, he joined the staff of the National Bureau of Standards, where he was employed until his retirement in 1998. As Chief of the NBS Atomic Spectroscopy Section from 1962 to 1998, he led the development of its reference data resources on the spectra of rare-earth elements, substantially increased its coverage of highly excited and ionized species, and pioneered the publication of NIST Standard Reference Data on the internet.

Katharine Blodgett Gebbie was an American astrophysicist and civil servant. She was the founding Director of the Physical Measurement Laboratory of the National Institute of Standards and Technology (NIST), and of its two immediate predecessors, the Physics Laboratory and the Center for Atomic, Molecular and Optical Physics, both for which she was the only Director. During her 22 years of management of these institutions, four of its scientists were awarded the Nobel Prize in Physics. In 2015, the NIST Katharine Blodgett Gebbie Laboratory Building in Boulder, Colorado was named in her honor.

Ernest Ambler was a British-American physicist who served as the Acting Under Secretary for Technology in the Department of Commerce (1988–89), as director of the United States' National Bureau of Standards, and as the first director of the United States' National Institute of Standards and Technology 1988–89.
Morris Cohen was a Canadian chemist working for the National Research Council of Canada in Ottawa. He contributed to the sciences of corrosion and of oxidation of metals.
Mars Guy Fontana was a corrosion engineer, professor of Metallurgical Engineering at Ohio State University. He was born April 6, 1910, in Iron Mountain, Michigan, and died February 29, 1988.
References
- ↑ Romanoff, Melvin (1964). "Exterior Corrosion of Cast-Iron Pipe". Journal AWWA. 56 (9): 1129–1143. doi:10.1002/j.1551-8833.1964.tb01314.x. ISSN 1551-8833.
- ↑ Schwerdtfeger, W J; Romanoff, Melvin (1972). "NBS papers on underground corrosion of steel piling 1962-1971". Gaithersburg, MD. doi: 10.6028/nbs.mono.127 .
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Romanoff, Melvin. Underground Corrosion.
- ↑ OFFICIAL EXHIBIT - ENT000391-00-BD01 - M. Romanoff, Underground Corrosion, National Bureau of Standards Circular (1957). (nrc.gov)
- ↑ "NIST Hall of Fame" (PDF).
- ↑ Romanoff, M., & United States. (1957). Underground corrosion. Washington, DC: United States Government Printing Office.
- ↑ "NBS PAPERS ON UNDERGROUND CORROSION OF STEEL PILING 1962-1971". trid.trb.org. Retrieved 2021-07-24.
- ↑ "NBS Papers on Underground Corrosion of Steel Piling" (PDF). NIST.gov. p. 4. LCCN 72-600037.