
A metallodendrimer is a type of dendrimer with incorporated metal atoms. The development of this type of material is actively pursued in academia. [1] [2] [3]

A metallodendrimer is a type of dendrimer with incorporated metal atoms. The development of this type of material is actively pursued in academia. [1] [2] [3]
The metal can be situated in the repeat unit, the core or at the extremities as end-group. Elements often encountered are palladium and platinum. These metals can form octahedral six-coordinate M(IV) linking units from organic dihalides and the corresponding 4-coordinate M(II) monomers. Ferrocene-containing dendrimers and dendrimers with cobaltocene and arylchromiumtricarbonyl units have been reported in end-functional dendrimers.
Metallodendrimers can form as metal complexes with dendritic counter ions for example by hydrolysis of ester terminated PAMAM dendrimers with sodium hydroxide.
Metallodendrimers are investigated as equivalents to nanoparticles. Applications can be expected in the fields of catalysis, as chemical sensors in molecular recognition - for example of bromine and chloride anions [4] - or as materials capable of binding metals. Metallodendrimers can also mimic certain biomolecules for example haemoprotein in dendrimer with a porphyrin core. Further uses are reported as electrocatalyst. [5] [6]
Examples of metallodendrimer heterogeneous catalysis are a nickel-containing dendrimer active in the Kharasch addition, [7] palladium-containing dendrimers active in ethylene polymerization [8] and in the Heck reaction. [9]

Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired by her when she slew Pallas. Palladium, platinum, rhodium, ruthenium, iridium and osmium form a group of elements referred to as the platinum group metals (PGMs). They have similar chemical properties, but palladium has the lowest melting point and is the least dense of them.
The Suzuki reaction is an organic reaction, classified as a cross-coupling reaction, where the coupling partners are a boronic acid and an organohalide and the catalyst is a palladium(0) complex. It was first published in 1979 by Akira Suzuki, and he shared the 2010 Nobel Prize in Chemistry with Richard F. Heck and Ei-ichi Negishi for their contribution to the discovery and development of palladium-catalyzed cross-couplings in organic synthesis. This reaction is also known as the Suzuki–Miyaura reaction or simply as the Suzuki coupling. It is widely used to synthesize polyolefins, styrenes, and substituted biphenyls. Several reviews have been published describing advancements and the development of the Suzuki reaction. The general scheme for the Suzuki reaction is shown below, where a carbon-carbon single bond is formed by coupling an organoboron species (R1-BY2) with a halide (R2-X) using a palladium catalyst and a base.
The Sonogashira reaction is a cross-coupling reaction used in organic synthesis to form carbon–carbon bonds. It employs a palladium catalyst as well as copper co-catalyst to form a carbon–carbon bond between a terminal alkyne and an aryl or vinyl halide.

Dendrimers are highly ordered, branched polymeric molecules. The name comes from the Czech word dheidri (deidri) which translates to "tree". Synonymous terms for dendrimer include arborols and cascade molecules. Typically, dendrimers are symmetric about the core, and often adopt a spherical three-dimensional morphology. The word dendron is also encountered frequently. A dendron usually contains a single chemically addressable group called the focal point or core. The difference between dendrons and dendrimers is illustrated in the top figure, but the terms are typically encountered interchangeably.
Organopalladium chemistry is a branch of organometallic chemistry that deals with organic palladium compounds and their reactions. Palladium is often used as a catalyst in the reduction of alkenes and alkynes with hydrogen. This process involves the formation of a palladium-carbon covalent bond. Palladium is also prominent in carbon-carbon coupling reactions, as demonstrated in tandem reactions.
Nanomaterial-based catalysts are usually heterogeneous catalysts broken up into metal nanoparticles in order to enhance the catalytic process. Metal nanoparticles have high surface area, which can increase catalytic activity. Nanoparticle catalysts can be easily separated and recycled. They are typically used under mild conditions to prevent decomposition of the nanoparticles.

In organometallic chemistry, a metallacycle is a derivative of a carbocyclic compound wherein a metal has replaced at least one carbon center; this is to some extent similar to heterocycles. Metallacycles appear frequently as reactive intermediates in catalysis, e.g. olefin metathesis and alkyne trimerization. In organic synthesis, directed ortho metalation is widely used for the functionalization of arene rings via C-H activation. One main effect that metallic atom substitution on a cyclic carbon compound is distorting the geometry due to the large size of typical metals.
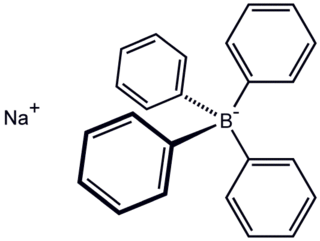
Sodium tetraphenylborate is the organic compound with the formula NaB(C6H5)4. It is a salt, wherein the anion consists of four phenyl rings bonded to boron. This white crystalline solid is used to prepare other tetraphenylborate salts, which are often highly soluble in organic solvents. The compound is used in inorganic and organometallic chemistry as a precipitating agent for potassium, ammonium, rubidium, and cesium ions, and some organic nitrogen compounds.
In chemistry, π-effects or π-interactions are a type of non-covalent interaction that involves π systems. Just like in an electrostatic interaction where a region of negative charge interacts with a positive charge, the electron-rich π system can interact with a metal, an anion, another molecule and even another π system. Non-covalent interactions involving π systems are pivotal to biological events such as protein-ligand recognition.
A cross-coupling reaction in organic chemistry is a reaction where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M reacts with an organic halide of the type R'-X with formation of a new carbon–carbon bond in the product R-R'. Cross-coupling reaction are a subset of coupling reactions. It is often used in arylations.

Established in 2005, Polymer Factory concentrates on developing well defined dendrimers and dendron based on 2,2-bis(methylol)propionic acid where the company has the exclusive right to the production, marketing, and sales of such materials. The company also provides tailor-made hyperbranched polymers. Polymer Factory's research lab is located in Stockholm, Sweden.

Didier Astruc carried out his studies in chemistry in Rennes. After a Ph. D. with professor R. Dabard in organometallic chemistry, he did post-doctoral studies with professor R. R. Schrock at the Massachusetts Institute of Technology Cambridge, Massachusetts, in the U.S. and later a sabbatical year with professor K. P. C. Vollhardt at the University of California at Berkeley. He became a CNRS Director of research in Rennes, then in 1983 full Professor of Chemistry at the University Bordeaux 1. He is known for his work on “Electron-Reservoir” complexes and dendritic molecular batteries, catalytic processes using nanoreactors and molecular recognition using gold nanoparticles and metallodendrimers. He is the author of three books, scientific publications and the editor of five books or special issues. He has been a member of the National CNRS committee from 2000 to 2008 and the President of the Coordination Chemistry Division of the Société Française de Chimie from 2000 to 2004. Didier Astruc is on the Thompson-Reuters list of the top 100 chemists who have achieved the highest citation impact scores for their chemistry papers published between 2000 and 2010. and on the list of the Highest Cited Researchers 2015 and 2016 (Thomson-Reuters). and 2017

Metal halides are compounds between metals and halogens. Some, such as sodium chloride are ionic, while others are covalently bonded. Covalently bonded metal halides may be discrete molecules, such as uranium hexafluoride, or they may form polymeric structures, such as palladium chloride.

In coordination chemistry phosphines are L-type ligands. Unlike most metal ammine complexes, metal phosphine complexes tend to be lipophilic, displaying good solubility in organic solvents. They also are compatible with metals in multiple oxidation states. Because of these two features, metal phosphine complexes are useful in homogeneous catalysis. Prominent examples of metal phosphine complexes include Wilkinson's catalyst (Rh(PPh3)3Cl), Grubbs' catalyst, and tetrakis(triphenylphosphine)palladium(0).

Half sandwich compounds are organometallic complexes that feature a cyclic polyhapto ligand bound to an MLn center, where L is a unidentate ligand. Thousands of such complexes are known. Well-known examples include cyclobutadieneiron tricarbonyl and (C5H5)TiCl3. Commercially useful examples include (C5H5)Co(CO)2, which is used in the synthesis of substituted pyridines, and methylcyclopentadienyl manganese tricarbonyl, an antiknock agent in petrol.

Phosphinooxazolines are a class of chiral ligands used in asymmetric catalysis. Their complexes are particularly effective at generating single enatiomers in reactions involving highly symmetric transition states, such as allylic substitutions, which are typically difficult to perform stereoselectively. The ligands are bidentate and have been shown to be hemilabile with the softer P‑donor being more firmly bound than the harder N‑donor.

In organometallic chemistry, palladium-NHC complexes are a family of organopalladium compounds in which palladium forms a coordination complex with N-Heterocyclic carbenes (NHCs). They have been investigated for applications in homogeneous catalysis, particularly cross-coupling reactions.

Transition-metal allyl complexes are coordination complexes with allyl and its derivatives as ligands. Allyl is the radical with the connectivity CH2CHCH2, although as a ligand it is usually viewed as an allyl anion CH2=CH−CH2−, which is usually described as two equivalent resonance structures.
In organometallic chemistry, palladacycle, as a class of metallacycles, refers to complexes containing at least one carbon-palladium bond. Palladacycles are invoked as intermediates in catalytic or palladium mediated reactions. They have been investigated as pre-catalysts for homogeneous catalysis and synthesis.
Concerted metalation-deprotonation (CMD) is a mechanistic pathway through which transition-metal catalyzed C–H activation reactions can take place. In a CMD pathway, the C–H bond of the substrate is cleaved and the new C–Metal bond forms through a single transition state. This process does not go through a metal species that is bound to the cleaved hydrogen atom. Instead, a carboxylate or carbonate base deprotonates the substrate. The first proposal of a concerted metalation deprotonation pathway was by S. Winstein and T. G. Traylor in 1955 for the acetolysis of diphenylmercury. It was found to be the lowest energy transition state in a number of computational studies, was experimentally confirmed through NMR experiments, and has been hypothesized to occur in mechanistic studies.