
Brownian motion is the random motion of particles suspended in a medium.

Molecular diffusion, often simply called diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size (mass) of the particles. Diffusion explains the net flux of molecules from a region of higher concentration to one of lower concentration. Once the concentrations are equal the molecules continue to move, but since there is no concentration gradient the process of molecular diffusion has ceased and is instead governed by the process of self-diffusion, originating from the random motion of the molecules. The result of diffusion is a gradual mixing of material such that the distribution of molecules is uniform. Since the molecules are still in motion, but an equilibrium has been established, the result of molecular diffusion is called a "dynamic equilibrium". In a phase with uniform temperature, absent external net forces acting on the particles, the diffusion process will eventually result in complete mixing.

Fick's laws of diffusion describe diffusion and were first posited by Adolf Fick in 1855 on the basis of largely experimental results. They can be used to solve for the diffusion coefficient, D. Fick's first law can be used to derive his second law which in turn is identical to the diffusion equation.
A viscometer is an instrument used to measure the viscosity of a fluid. For liquids with viscosities which vary with flow conditions, an instrument called a rheometer is used. Thus, a rheometer can be considered as a special type of viscometer. Viscometers can measure only constant viscosity, that is, viscosity that does not change with flow conditions.

In industrial process engineering, mixing is a unit operation that involves manipulation of a heterogeneous physical system with the intent to make it more homogeneous. Familiar examples include pumping of the water in a swimming pool to homogenize the water temperature, and the stirring of pancake batter to eliminate lumps (deagglomeration).
Electrical mobility is the ability of charged particles to move through a medium in response to an electric field that is pulling them. The separation of ions according to their mobility in gas phase is called ion mobility spectrometry, in liquid phase it is called electrophoresis.
Fluorescence correlation spectroscopy (FCS) is a statistical analysis, via time correlation, of stationary fluctuations of the fluorescence intensity. Its theoretical underpinning originated from L. Onsager's regression hypothesis. The analysis provides kinetic parameters of the physical processes underlying the fluctuations. One of the interesting applications of this is an analysis of the concentration fluctuations of fluorescent particles (molecules) in solution. In this application, the fluorescence emitted from a very tiny space in solution containing a small number of fluorescent particles (molecules) is observed. The fluorescence intensity is fluctuating due to Brownian motion of the particles. In other words, the number of the particles in the sub-space defined by the optical system is randomly changing around the average number. The analysis gives the average number of fluorescent particles and average diffusion time, when the particle is passing through the space. Eventually, both the concentration and size of the particle (molecule) are determined. Both parameters are important in biochemical research, biophysics, and chemistry.
A single-molecule experiment is an experiment that investigates the properties of individual molecules. Single-molecule studies may be contrasted with measurements on an ensemble or bulk collection of molecules, where the individual behavior of molecules cannot be distinguished, and only average characteristics can be measured. Since many measurement techniques in biology, chemistry, and physics are not sensitive enough to observe single molecules, single-molecule fluorescence techniques caused a lot of excitement, since these supplied many new details on the measured processes that were not accessible in the past. Indeed, since the 1990s, many techniques for probing individual molecules have been developed.
Surface photovoltage (SPV) measurements are a widely used method to determine the minority carrier diffusion length of semiconductors. Since the transport of minority carriers determines the behavior of the p-n junctions that are ubiquitous in semiconductor devices, surface photovoltage data can be very helpful in understanding their performance. As a contactless method, SPV is a popular technique for characterizing poorly understood compound semiconductors where the fabrication of ohmic contacts or special device structures may be difficult.
In biology, membrane fluidity refers to the viscosity of the lipid bilayer of a cell membrane or a synthetic lipid membrane. Lipid packing can influence the fluidity of the membrane. Viscosity of the membrane can affect the rotation and diffusion of proteins and other bio-molecules within the membrane, there-by affecting the functions of these things.

Rotational diffusion is the rotational movement which acts upon any object such as particles, molecules, atoms when present in a fluid, by random changes in their orientations. Whilst the directions and intensities of these changes are statistically random, they do not arise randomly and are instead the result of interactions between particles. One example occurs in colloids, where relatively large insoluble particles are suspended in a greater amount of fluid. The changes in orientation occur from collisions between the particle and the many molecules forming the fluid surrounding the particle, which each transfer kinetic energy to the particle, and as such can be considered random due to the varied speeds and amounts of fluid molecules incident on each individual particle at any given time.
Fluorescence cross-correlation spectroscopy (FCCS) is a spectroscopic technique that examines the interactions of fluorescent particles of different colours as they randomly diffuse through a microscopic detection volume over time, under steady conditions.
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water. Viscosity is defined scientifically as a force multiplied by a time divided by an area. Thus its SI units are newton-seconds per square meter, or pascal-seconds.

Gas is one of the four fundamental states of matter. The others are solid, liquid, and plasma. A pure gas may be made up of individual atoms, elemental molecules made from one type of atom, or compound molecules made from a variety of atoms. A gas mixture, such as air, contains a variety of pure gases. What distinguishes gases from liquids and solids is the vast separation of the individual gas particles. This separation usually makes a colorless gas invisible to the human observer.
The convection–diffusion equation is a combination of the diffusion and convection (advection) equations, and describes physical phenomena where particles, energy, or other physical quantities are transferred inside a physical system due to two processes: diffusion and convection. Depending on context, the same equation can be called the advection–diffusion equation, drift–diffusion equation, or (generic) scalar transport equation.

Diffusion is the net movement of anything generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical potential. It is possible to diffuse "uphill" from a region of lower concentration to a region of higher concentration, as in spinodal decomposition. Diffusion is a stochastic process due to the inherent randomness of the diffusing entity and can be used to model many real-life stochastic scenarios. Therefore, diffusion and the corresponding mathematical models are used in several fields beyond physics, such as statistics, probability theory, information theory, neural networks, finance, and marketing.
Lipid bilayer characterization is the use of various optical, chemical and physical probing methods to study the properties of lipid bilayers. Many of these techniques are elaborate and require expensive equipment because the fundamental nature of the lipid bilayer makes it a very difficult structure to study. An individual bilayer, since it is only a few nanometers thick, is invisible in traditional light microscopy. The bilayer is also a relatively fragile structure since it is held together entirely by non-covalent bonds and is irreversibly destroyed if removed from water. In spite of these limitations dozens of techniques have been developed over the last seventy years to allow investigations of the structure and function of bilayers. The first general approach was to utilize non-destructive in situ measurements such as x-ray diffraction and electrical resistance which measured bilayer properties but did not actually image the bilayer. Later, protocols were developed to modify the bilayer and allow its direct visualization at first in the electron microscope and, more recently, with fluorescence microscopy. Over the past two decades, a new generation of characterization tools including AFM has allowed the direct probing and imaging of membranes in situ with little to no chemical or physical modification. More recently, dual polarisation interferometry has been used to measure the optical birefringence of lipid bilayers to characterise order and disruption associated with interactions or environmental effects.
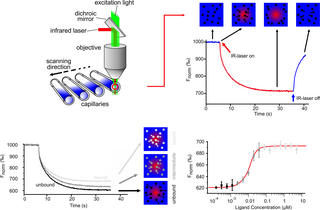
Microscale thermophoresis (MST) is a technology for the biophysical analysis of interactions between biomolecules. Microscale thermophoresis is based on the detection of a temperature-induced change in fluorescence of a target as a function of the concentration of a non-fluorescent ligand. The observed change in fluorescence is based on two distinct effects. On the one hand it is based on a temperature related intensity change (TRIC) of the fluorescent probe, which can be affected by binding events. On the other hand, it is based on thermophoresis, the directed movement of particles in a microscopic temperature gradient. Any change of the chemical microenvironment of the fluorescent probe, as well as changes in the hydration shell of biomolecules result in a relative change of the fluorescence detected when a temperature gradient is applied and can be used to determine binding affinities. MST allows measurement of interactions directly in solution without the need of immobilization to a surface.
In engineering, physics, and chemistry, the study of transport phenomena concerns the exchange of mass, energy, charge, momentum and angular momentum between observed and studied systems. While it draws from fields as diverse as continuum mechanics and thermodynamics, it places a heavy emphasis on the commonalities between the topics covered. Mass, momentum, and heat transport all share a very similar mathematical framework, and the parallels between them are exploited in the study of transport phenomena to draw deep mathematical connections that often provide very useful tools in the analysis of one field that are directly derived from the others.
Force Spectrum Microscopy (FSM) is an application of active microrheology developed to measure aggregate random forces in the cytoplasm. Large, inert flow tracers are injected into live cells and become lodged inside the cytoskeletal mesh, wherein it is oscillated by repercussions from active motor proteins. The magnitude of these random forces can be inferred from the frequency of oscillation of tracer particles. Tracking the fluctuations of tracer particles using optical microscopy can isolate the contribution of active random forces to intracellular molecular transport from that of Brownian motion.








