Related Research Articles

RDX (abbreviation of "Research Department eXplosive" or Royal Demolition eXplosive) or hexogen, among other names, is an organic compound with the formula (CH2N2O2)3. It is white, odorless, and tasteless, widely used as an explosive. Chemically, it is classified as a nitroamine alongside HMX, which is a more energetic explosive than TNT. It was used widely in World War II and remains common in military applications.

An explosive is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An explosive charge is a measured quantity of explosive material, which may either be composed solely of one ingredient or be a mixture containing at least two substances.

Trinitrotoluene, more commonly known as TNT (and more specifically 2,4,6-trinitrotoluene, and by its preferred IUPAC name 2-methyl-1,3,5-trinitrobenzene), is a chemical compound with the formula C6H2(NO2)3CH3. TNT is occasionally used as a reagent in chemical synthesis, but it is best known as an explosive material with convenient handling properties. The explosive yield of TNT is considered to be the standard comparative convention of bombs and asteroid impacts. In chemistry, TNT is used to generate charge transfer salts.

A solid-propellant rocket or solid rocket is a rocket with a rocket engine that uses solid propellants (fuel/oxidizer). The earliest rockets were solid-fuel rockets powered by gunpowder. The inception of gunpowder rockets in warfare can be credited to the ancient Chinese, and in the 13th century, the Mongols played a pivotal role in facilitating their westward adoption.

2,4,6-Trinitrophenylmethylnitramine or tetryl (C7H5N5O8) is an explosive compound used to make detonators and explosive booster charges.

Ammonium nitrate is a chemical compound with the formula NH4NO3. It is a white crystalline salt consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, although it does not form hydrates. It is predominantly used in agriculture as a high-nitrogen fertilizer.

Amatol is a highly explosive material made from a mixture of TNT and ammonium nitrate. The British name originates from the words ammonium and toluene. Similar mixtures were known as Schneiderite in France. Amatol was used extensively during World War I and World War II, typically as an explosive in military weapons such as aircraft bombs, shells, depth charges, and naval mines. It was eventually replaced with alternative explosives such as Composition B, Torpex, and Tritonal.

A shell, in a modern military context, is a projectile whose payload contains an explosive, incendiary, or other chemical filling. Originally it was called a bombshell, contrasting with solid shells used for early rifled artillery, but "shell" has come to be unambiguous in a military context. A shell can hold a tracer.

Tritonal is a mixture of 80% TNT and 20% aluminium powder, used in several types of ordnance such as air-dropped bombs. The aluminium increases the total heat output and hence impulse of the TNT – the length of time during which the blast wave is positive. Tritonal is approximately 18% more powerful than TNT alone.

Ammonal is an explosive made up of ammonium nitrate and aluminium powder. TNT is added to create T-ammonal which improves properties such as brisance. The mixture is often referred to as Tannerite, which is a brand of ammonal.

Flash powder is a pyrotechnic composition, a mixture of oxidizer and metallic fuel, which burns quickly (deflagrates) and produces a loud noise regardless of confinement. It is widely used in theatrical pyrotechnics and fireworks and was once used for flashes in photography.

Torpex is a secondary explosive, 50% more powerful than TNT by mass. Torpex comprises 42% RDX, 40% TNT and 18% powdered aluminium. It was used in the Second World War from late 1942, at which time some used the names Torpex and RDX interchangeably, much to the confusion of today's historical researchers. Torpex proved to be particularly useful in underwater munitions because the aluminium component made the explosive pulse last longer, which increased the destructive power. Besides torpedoes, naval mines, and depth charges, Torpex was only used in the Upkeep, Tallboy and Grand Slam bombs as well as the drones employed in Operation Aphrodite. Torpex has long been superseded by H6 and Polymer-bonded explosive (PBX) compositions. It is regarded as obsolete and Torpex is unlikely to be encountered except in old munitions or unexploded ordnance, although a notable exception to this is the Sting Ray lightweight torpedo, which as of October 2020 remains in service with the Royal Navy and several foreign militaries. The German equivalent of Torpex was Trialen.
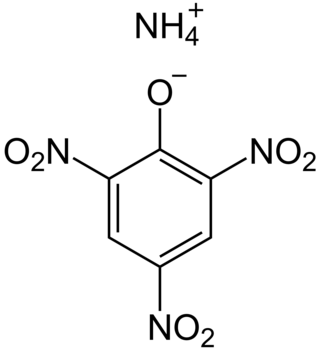
Dunnite, also known as Explosive D or systematically as ammonium picrate, is an explosive developed in 1906 by US Army Major Beverly W. Dunn, who later served as chief inspector of the Bureau of Transportation Explosives. Ammonium picrate is a salt formed by reacting picric acid and ammonia. It is chemically related to the more stable explosive trinitrotoluene (TNT).
A pyrotechnic composition is a substance or mixture of substances designed to produce an effect by heat, light, sound, gas/smoke or a combination of these, as a result of non-detonative self-sustaining exothermic chemical reactions. Pyrotechnic substances do not rely on oxygen from external sources to sustain the reaction.
Composition H-6 is a melt-cast military aluminized high explosive. H-6 was developed in the United States.
Oxygen balance is an expression that is used to indicate the degree to which an explosive can be oxidized, to determine if an explosive molecule contains enough oxygen to fully oxidize the other atoms in the explosive. For example, fully oxidized carbon forms carbon dioxide, hydrogen forms water, sulfur forms sulfur dioxide, and metals form metal oxides. A molecule is said to have a positive oxygen balance if it contains more oxygen than is needed and a negative oxygen balance if it contains less oxygen than is needed.
Hexanite was a castable German military explosive developed early in the 20th century before the First World War for the Kaiserliche Marine, intended to augment supplies of trinitrotoluene (TNT), which were then in short supply. Hexanite is slightly less powerful than TNT on its own. The most common hexanite formula was 60% TNT and 40% hexanitrodiphenylamine.
Explosive materials are produced in numerous physical forms for their use in mining, engineering, or military applications. The different physical forms and fabrication methods are grouped together in several use forms of explosives.

A water-gel explosive is a fuel-sensitized explosive mixture consisting of an aqueous ammonium nitrate solution that acts as the oxidizer. Water gels that are cap-insensitive are referred to under United States safety regulations as blasting agents. Water gel explosives have a jelly-like consistency and come in sausage-like packing stapled shut on both sides.
References
- 1 2 3 4 5 J. Hendrikson (January 1, 1978). "Minol". Encyclopedia of explosives and related items. By Seymour M. Kaye. Vol. 8. Picatinny Arsenal. pp. M135 –M143. LCCN 61-61759.
- ↑ Boyars, Carl; Holden, James R.; Bertram, Albert L. (March 29, 1973). Minol IV, A New Explosive Composition Containing Ammonium Nitrate-Potassium Nitrate Solid Solution. Maryland: Naval Ordnance Laboratory.