A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and plus (+) and minus (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name, and it contains no words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulas can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than are chemical names and structural formulas.

Scandium is a chemical element with symbol Sc and atomic number 21. A silvery-white metallic d-block element, it has historically been classified as a rare-earth element, together with yttrium and the lanthanides. It was discovered in 1879 by spectral analysis of the minerals euxenite and gadolinite from Scandinavia.

Scandium(III) chloride is the inorganic compound with the formula ScCl3. It is a white, high-melting ionic compound, which is deliquescent and highly water-soluble. Scandium(III) chloride is mainly of interest in the research laboratory. Both the anhydrous form and hexahydrate (ScCl3•6H2O) are commercially available.
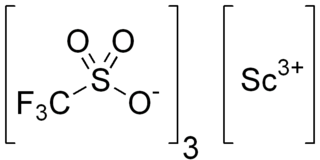
Scandium trifluoromethanesulfonate, commonly called scandium triflate, is a chemical compound with formula Sc(SO3CF3)3, a salt consisting of scandium cations Sc3+ and triflate SO3CF3− anions.

Scandium(III) fluoride, ScF3, is an ionic compound. It is slightly soluble in water but dissolves in the presence of excess fluoride to form the ScF63− anion.

Titanium(II) sulfide (TiS) is an inorganic chemical compound of titanium and sulfur.

Scandium(III) sulfide is a chemical compound of scandium and sulfur with the chemical formula Sc2S3. It is a yellow solid.
Copper sulfides describe a family of chemical compounds and minerals with the formula CuSy. Both minerals and synthetic materials comprise these compounds. Some copper sulfides are economically important ores.
Organoscandium chemistry is the chemistry of organometallic compounds containing a carbon to scandium chemical bond. The interest in organoscandium compounds is mostly academic but several compound classes find practical application in catalysis, especially in polymerization. A common precursor is scandium chloride.

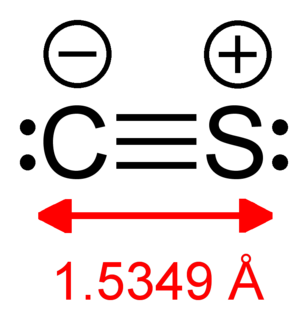
Scandium monosulfide is a chemical compound of scandium and sulfur with the chemical formula ScS. Although its formula might suggest that it is a compound of scandium(II), i.e. [Sc2+][S2−], ScS is probably more realistically described as a pseudo-ionic compound, containing [Sc3+][S2−], with the remaining electron occupying the conduction band of the solid.
Scandium trihydride is an unstable molecular chemical compound with the chemical formula ScH3. It has been formed as one of a number of other molecular scandium hydride products at low temperature using laser ablation and identified by infrared spectroscopy.
Scandium trihydride has recently been the subject of Dirac–Hartree–Fock relativistic calculation studies, which investigate the stabilities, geometries, and relative energies of hydrides of the formula MH3, MH2, or MH.
Samarium monochalcogenides are chemical compounds with the composition SmX, where Sm stands for the lanthanide element samarium and X denotes any one of three chalcogen elements, sulfur, selenium or tellurium, resulting in the compounds SmS, SmSe or SmTe. In these compounds, samarium formally exhibits oxidation state +2, whereas it usually assumes the +3 state, resulting in chalcogenides with the chemical formula Sm2X3.
Scandium sulfide can refer to two different sulfides of scandium:
Uranium monosulfide is an inorganic chemical compound of uranium and sulfur.
Germanium monosulfide or Germanium(II) sulfide is the chemical compound with the formula GeS. It is a chalcogenide glass and a semiconductor. Germanium sulfide is described as a red-brown powder or black crystals. Germanium(II) sulfide when dry is stable in air, hydrolyzes slowly in moist air but rapidly reacts in water forming Ge(OH)2 and then GeO. It is one of a few sulfides that can be sublimed under vacuum without decomposition.
Scandium sulfate is the scandium salt of sulfuric acid and has the formula Sc2(SO4)3. It is used in agriculture as a very dilute solution as a seed treatment to improve the germination of corn, peas, wheat, and other plants.

Scandium triiodide, also known as scandium iodide, is an inorganic compound with the formula ScI3 and is classified as a lanthanide iodide. It is a yellowish powder. It is used in metal halide lamps together with similar compounds, such as caesium iodide, because of their ability to maximize emission of UV and to prolong bulb life. The maximized UV emission can be tuned to a range that can initiate photopolymerizations.
Scandium bromide, or ScBr3, is a trihalide, hygroscopic, water-soluble chemical compound of scandium and bromine.