
In cell biology, mitosis is a part of the cell cycle in which replicated chromosomes are separated into two new nuclei. Cell division by mitosis gives rise to genetically identical cells in which the total number of chromosomes is maintained. Therefore, mitosis is also known as equational division. In general, mitosis is preceded by S phase of interphase and is often followed by telophase and cytokinesis; which divides the cytoplasm, organelles and cell membrane of one cell into two new cells containing roughly equal shares of these cellular components. The different stages of mitosis altogether define the mitotic (M) phase of a cell cycle—the division of the mother cell into two daughter cells genetically identical to each other.

In cell biology, the centrosome is an organelle that serves as the main microtubule organizing center (MTOC) of the animal cell, as well as a regulator of cell-cycle progression. The centrosome provides structure for the cell. The centrosome is thought to have evolved only in the metazoan lineage of eukaryotic cells. Fungi and plants lack centrosomes and therefore use other structures to organize their microtubules. Although the centrosome has a key role in efficient mitosis in animal cells, it is not essential in certain fly and flatworm species.
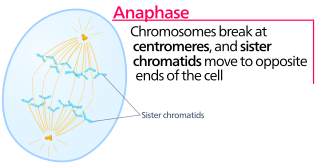
Anaphase is the stage of mitosis after the process of metaphase, when replicated chromosomes are split and the newly-copied chromosomes are moved to opposite poles of the cell. Chromosomes also reach their overall maximum condensation in late anaphase, to help chromosome segregation and the re-formation of the nucleus.

In cell biology, the spindle apparatus is the cytoskeletal structure of eukaryotic cells that forms during cell division to separate sister chromatids between daughter cells. It is referred to as the mitotic spindle during mitosis, a process that produces genetically identical daughter cells, or the meiotic spindle during meiosis, a process that produces gametes with half the number of chromosomes of the parent cell.

The spindle checkpoint, also known as the metaphase-to-anaphase transition, the spindle assembly checkpoint (SAC), the metaphase checkpoint, or the mitotic checkpoint, is a cell cycle checkpoint during mitosis or meiosis that prevents the separation of the duplicated chromosomes (anaphase) until each chromosome is properly attached to the spindle. To achieve proper segregation, the two kinetochores on the sister chromatids must be attached to opposite spindle poles. Only this pattern of attachment will ensure that each daughter cell receives one copy of the chromosome. The defining biochemical feature of this checkpoint is the stimulation of the anaphase-promoting complex by M-phase cyclin-CDK complexes, which in turn causes the proteolytic destruction of cyclins and proteins that hold the sister chromatids together.

A kinetochore is a disc-shaped protein structure associated with duplicated chromatids in eukaryotic cells where the spindle fibers attach during cell division to pull sister chromatids apart. The kinetochore assembles on the centromere and links the chromosome to microtubule polymers from the mitotic spindle during mitosis and meiosis. The term kinetochore was first used in a footnote in a 1934 Cytology book by Lester W. Sharp and commonly accepted in 1936. Sharp's footnote reads: "The convenient term kinetochore has been suggested to the author by J. A. Moore", likely referring to John Alexander Moore who had joined Columbia University as a freshman in 1932.
Aurora kinases are serine/threonine kinases that are essential for cell proliferation. They are phosphotransferase enzymes that help the dividing cell dispense its genetic materials to its daughter cells. More specifically, Aurora kinases play a crucial role in cellular division by controlling chromatid segregation. Defects in this segregation can cause genetic instability, a condition which is highly associated with tumorigenesis. The first aurora kinases were identified in Drosophila melanogaster, where mutations led to failure of centrosome separation with the monopolar spindles reminiscent of the North Pole, suggesting the name aurora.

Aurora kinase A also known as serine/threonine-protein kinase 6 is an enzyme that in humans is encoded by the AURKA gene.

Aurora kinase B is a protein that functions in the attachment of the mitotic spindle to the centromere.

Aurora kinase inhibitors are a putative drug class for treating cancer. The Aurora kinase enzymes could be potential targets for novel small-molecule enzyme inhibitors.
David Moore Glover is a British geneticist and Research Professor of Biology and Biological Engineering at the California Institute of Technology. He served as Balfour Professor of Genetics at the University of Cambridge, a Wellcome Trust investigator in the Department of Genetics at the University of Cambridge, and Fellow of Fitzwilliam College, Cambridge. He serves as the first editor-in-chief of the open-access journal Open Biology published by the Royal Society.
Anaphase lag is a consequence of an event during cell division where sister chromatids do not properly separate from each other because of improper spindle formation. The chromosome or chromatid does not properly migrate during anaphase and the daughter cells will lose some genetic information. It is one of many causes of aneuploidy. This event can occur during both meiosis and mitosis with unique repercussions. In either case, anaphase lag will cause one daughter cell to receive a complete set of chromosomes while the other lacks one paired set of chromosomes, creating a form of monosomy. Whether the cell survives depends on which sister chromatid was lost and the background genomic state of the cell. The passage of abnormal numbers of chromosomes will have unique consequences with regards to mosaicism and development as well as the progression and heterogeneity of cancers.

Serine/threonine-protein kinase PLK1, also known as polo-like kinase 1 (PLK-1) or serine/threonine-protein kinase 13 (STPK13), is an enzyme that in humans is encoded by the PLK1 gene.

Mitotic checkpoint serine/threonine-protein kinase BUB1 also known as BUB1 is an enzyme that in humans is encoded by the BUB1 gene.

Targeting protein for Xklp2 is a protein that in humans is encoded by the TPX2 gene. It is one of the many spindle assembly factors that play a key role in inducing microtubule assembly and growth during M phase.

Large tumor suppressor kinase 2 (LATS2) is an enzyme that in humans is encoded by the LATS2 gene.

Aurora kinase C, also Serine/threonine-protein kinase 13 is an enzyme that in humans is encoded by the AURKC gene.

Mitotic catastrophe has been defined as either a cellular mechanism to prevent potentially cancerous cells from proliferating or as a mode of cellular death that occurs following improper cell cycle progression or entrance. Mitotic catastrophe can be induced by prolonged activation of the spindle assembly checkpoint, errors in mitosis, or DNA damage and functioned to prevent genomic instability. It is a mechanism that is being researched as a potential therapeutic target in cancers, and numerous approved therapeutics induce mitotic catastrophe.
Biorientation is the phenomenon whereby microtubules emanating from different microtubule organizing centres (MTOCs) attach to kinetochores of sister chromatids. This results in the sister chromatids moving to opposite poles of the cell during cell division, and thus results in both daughter cells having the same genetic information.

Centrosomes are the major microtubule organizing centers (MTOC) in mammalian cells. Failure of centrosome regulation can cause mistakes in chromosome segregation and is associated with aneuploidy. A centrosome is composed of two orthogonal cylindrical protein assemblies, called centrioles, which are surrounded by a protein dense amorphous cloud of pericentriolar material (PCM). The PCM is essential for nucleation and organization of microtubules. The centrosome cycle is important to ensure that daughter cells receive a centrosome after cell division. As the cell cycle progresses, the centrosome undergoes a series of morphological and functional changes. Initiation of the centrosome cycle occurs early in the cell cycle in order to have two centrosomes by the time mitosis occurs.
















