Related Research Articles
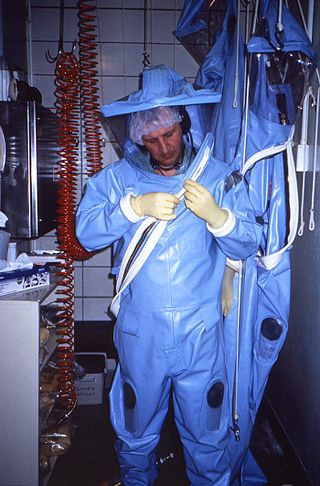
Biosafety is the prevention of large-scale loss of biological integrity, focusing both on ecology and human health. These prevention mechanisms include conduction of regular reviews of the biosafety in laboratory settings, as well as strict guidelines to follow. Biosafety is used to protect from harmful incidents. Many laboratories handling biohazards employ an ongoing risk management assessment and enforcement process for biosafety. Failures to follow such protocols can lead to increased risk of exposure to biohazards or pathogens. Human error and poor technique contribute to unnecessary exposure and compromise the best safeguards set into place for protection.
Biosecurity refers to measures aimed at preventing the introduction and/or spread of harmful organisms to animals and plants in order to minimize the risk of transmission of infectious disease. In agriculture, these measures are aimed at protecting food crops and livestock from pests, invasive species, and other organisms not conducive to the welfare of the human population. The term includes biological threats to people, including those from pandemic diseases and bioterrorism. The definition has sometimes been broadened to embrace other concepts, and it is used for different purposes in different contexts.
Health Canada is the department of the Government of Canada responsible for national health policy. The department itself is also responsible for numerous federal health-related agencies, including the Canadian Food Inspection Agency (CFIA) and the Public Health Agency of Canada (PHAC), among others. These organizations help to ensure compliance with federal law in a variety of healthcare, agricultural, and pharmaceutical activities. This responsibility also involves extensive collaboration with various other federal- and provincial-level organizations in order to ensure the safety of food, health, and pharmaceutical products—including the regulation of health research and pharmaceutical manufacturing/testing facilities.
In general, compliance means conforming to a rule, such as a specification, policy, standard or law. Compliance has traditionally been explained by reference to the deterrence theory, according to which punishing a behavior will decrease the violations both by the wrongdoer and by others. This view has been supported by economic theory, which has framed punishment in terms of costs and has explained compliance in terms of a cost-benefit equilibrium. However, psychological research on motivation provides an alternative view: granting rewards or imposing fines for a certain behavior is a form of extrinsic motivation that weakens intrinsic motivation and ultimately undermines compliance.
Law enforcement in Malaysia is performed by numerous law enforcement agencies and primarily the responsibility of the Royal Malaysia Police. Like many federal nations, the nature of the Constitution of Malaysia mandates law and order as a subject of a state, which means that local government bodies also have a role to play in law enforcement, therefore the bulk of the policing lies with the respective states and territories of Malaysia. Below are some of the law enforcement bodies and agencies of Malaysia.

The National Agency for Food and Drug Administration and Control(NAFDAC) in Nigeria is a federal agency under the Federal Ministry of Health that is responsible for regulating and controlling the manufacture, importation, exportation, advertisement, distribution, sale and use of food, drugs, cosmetics, medical devices, chemicals and packaged water.

One use of the concept of biocontainment is related to laboratory biosafety and pertains to microbiology laboratories in which the physical containment of pathogenic organisms or agents is required, usually by isolation in environmentally and biologically secure cabinets or rooms, to prevent accidental infection of workers or release into the surrounding community during scientific research.

Food safety in China is a concern relating to agriculture in the world's most populated country. China's principal crops are rice, corn, wheat, soybeans, and cotton in addition to apples and other fruits and vegetables. China's principal livestock products include pork, beef, dairy, and eggs. The Chinese government oversees agricultural production as well as the manufacture of food packaging, containers, chemical additives, drug production, and business regulation. In recent years, the Chinese government attempted to consolidate food safety regulation with the creation of the State Food and Drug Administration of China in 2003; officials have also been under increasing public and international pressure to solve food safety problems. Chinese Vice Premier Li Keqiang said, "Food is essential, and safety should be a top priority. Food safety is closely related to people's lives and health and economic development and social harmony," at a State Council meeting in Beijing.

The National Institute of Technology and Evaluation, or NITE is an Independent Administrative Institution, established by Japanese government, aiming to contribute to the "safety and security of life" supported by reliable technology and information.

The regulation of genetic engineering varies widely by country. Countries such as the United States, Canada, Lebanon and Egypt use substantial equivalence as the starting point when assessing safety, while many countries such as those in the European Union, Brazil and China authorize GMO cultivation on a case-by-case basis. Many countries allow the import of GM food with authorization, but either do not allow its cultivation or have provisions for cultivation, but no GM products are yet produced. Most countries that do not allow for GMO cultivation do permit research. Most (85%) of the world's GMO crops are grown in the Americas. One of the key issues concerning regulators is whether GM products should be labeled. Labeling of GMO products in the marketplace is required in 64 countries. Labeling can be mandatory up to a threshold GM content level or voluntary. A study investigating voluntary labeling in South Africa found that 31% of products labeled as GMO-free had a GM content above 1.0%. In Canada and the USA labeling of GM food is voluntary, while in Europe all food or feed which contains greater than 0.9% of approved GMOs must be labelled.
National biosecurity in Australia is governed and administered by two federal government departments, the Department of Health and the Department of Agriculture, Fisheries and Forestry. The Biosecurity Act 2015 (C'wealth) and related legislation is administered by the two departments and manages biosecurity risks at the national border. The Act aims to manage biosecurity risks to human health, agriculture, native flora and fauna and the environment. It also covers Australia's international rights and obligations, and lists specific diseases which are contagious and capable of causing severe harm to human health. Each state and territory has additional legislation and protocols to cover biosecurity in their jurisdiction (post-border) including the detection of pests and diseases that have breached the national border.
Biosecurity in the United States is governed by the Bureau of Western Hemisphere Affairs, which is part of the US Department of State. It obtains guidance and advice on specific matters relating to biosecurity from various other government agencies.
Food safety in Australia concerns the production, distribution, preparation, and storage of food in Australia to prevent foodborne illness, also known as food safety. Food Standards Australia New Zealand is responsible for developing food standards for Australia and New Zealand.

The National Environmental Standards and Regulations Enforcement Agency is an environmental agency of the Federal Government of Nigeria that was established by law in 2007 to "ensure a cleaner and healthier environment for Nigerians". The agency functions as a parastatal enterprise of the Federal Ministry of Environment, and it is headed by a director general, who is also the chief executive officer of about 483 companies in the NESREA corporate family. Human activities that have negative effects on the environment are covered by NESREA's 33 National Environmental Regulations. The agency's authority includes process and equipment monitoring, compliance with set standards, disciplining violators of set rules, conducting public investigations, and submission of proposals to the minister for review in order to maintain environmental quality.
NIH Office of Science Policy is the primary advisor to the Director of the NIH on matters of biomedical research policy issues that are of significance to the agency, the research community, and the public. The office also works with stakeholders within and outside of NIH to develop policies that promote progress in the life sciences. The current Acting NIH Associate Director for Science Policy and Acting Director of the NIH Office of Science Policy is Lyric Jorgenson, Ph.D.
India and China are the two largest producers of genetically modified products in Asia. India currently only grows GM cotton, while China produces GM varieties of cotton, poplar, petunia, tomato, papaya and sweet pepper. Cost of enforcement of regulations in India are generally higher, possibly due to the greater influence farmers and small seed firms have on policy makers, while the enforcement of regulations was more effective in China. Other Asian countries that grew GM crops in 2011 were Pakistan, the Philippines and Myanmar. GM crops were approved for commercialisation in Bangladesh in 2013 and in Vietnam and Indonesia in 2014.
The hazards of synthetic biology include biosafety hazards to workers and the public, biosecurity hazards stemming from deliberate engineering of organisms to cause harm, and hazards to the environment. The biosafety hazards are similar to those for existing fields of biotechnology, mainly exposure to pathogens and toxic chemicals; however, novel synthetic organisms may have novel risks. For biosecurity, there is concern that synthetic or redesigned organisms could theoretically be used for bioterrorism. Potential biosecurity risks include recreating known pathogens from scratch, engineering existing pathogens to be more dangerous, and engineering microbes to produce harmful biochemicals. Lastly, environmental hazards include adverse effects on biodiversity and ecosystem services, including potential changes to land use resulting from agricultural use of synthetic organisms.
The Australian Department of Agriculture, Water and the Environment (DAWE) was an Australian Government department which operated from 1 February 2020 until 30 June 2022. It represented Australia's national interests in agriculture, water and the environment.
The Federal Ministry of Environment is a ministry of the Federal Government of Nigeria created in 1999 with a mandate to address environmental issues and to ensure the effective coordination of all environmental matters in the country. As part of its vision and mission, the ministry sets out to ensure the control of environmental issues and the protection and conservation of natural resources. It also formulates policies and supervises activities for curbing desertification and deforestation, the management of flood, erosion and pollution, as well as climate change and clean energy.
References
- ↑ eribake, akintayo (2015-04-26). "Nigeria gets biosafety law, joins league of biotech countries". Vanguard News. Retrieved 2022-09-10.
- ↑ "Jonathan signs biosafety bill into law". The Guardian Nigeria News. 2015-04-23. Retrieved 2022-09-10.
- ↑ "NBMA – Ensuring Proper Regulation of Modern Biotechnology and Providing Biosecurity" . Retrieved 2022-09-10.
- ↑ HOMEf (2020-10-29). "A Bill for an ACT to amend the National Biosafety Management Agency (Amendment) ACT, 2019". Health of Mother Earth Foundation. Retrieved 2022-09-10.
- ↑ "NBMA Boss seeks collaboration with Police on GMO regulation". The Guardian Nigeria News. 2017-10-01. Retrieved 2022-09-10.
- ↑ "NBMA seeks collaboration with interpol on biosafety permit". The Guardian Nigeria News. 2018-06-03. Retrieved 2022-09-10.
- ↑ "National biosafety agency trains 50 scientists". Tribune Online. 2022-08-02. Retrieved 2022-09-10.
- ↑ "At national conference, NBMA tasks FG on safe, modern biotechnology sustenance". The Guardian Nigeria News. 2019-12-29. Retrieved 2022-09-10.
- ↑ "NBMA drafts guidelines for genome editing". The Guardian Nigeria News. 2019-10-11. Retrieved 2022-09-10.