Evidence-based medicine (EBM) is "the conscientious, explicit and judicious use of current best evidence in making decisions about the care of individual patients." The aim of EBM is to integrate the experience of the clinician, the values of the patient, and the best available scientific information to guide decision-making about clinical management. The term was originally used to describe an approach to teaching the practice of medicine and improving decisions by individual physicians about individual patients.
Health Level Seven, abbreviated to HL7, is a range of global standards for the transfer of clinical and administrative health data between applications with the aim to improve patient outcomes and health system performance. The HL7 standards focus on the application layer, which is "layer 7" in the Open Systems Interconnection model. The standards are produced by Health Level Seven International, an international standards organization, and are adopted by other standards issuing bodies such as American National Standards Institute and International Organization for Standardization. There are a range of primary standards that are commonly used across the industry, as well as secondary standards which are less frequently adopted.

A medical guideline is a document with the aim of guiding decisions and criteria regarding diagnosis, management, and treatment in specific areas of healthcare. Such documents have been in use for thousands of years during the entire history of medicine. However, in contrast to previous approaches, which were often based on tradition or authority, modern medical guidelines are based on an examination of current evidence within the paradigm of evidence-based medicine. They usually include summarized consensus statements on best practice in healthcare. A healthcare provider is obliged to know the medical guidelines of their profession, and has to decide whether to follow the recommendations of a guideline for an individual treatment.

The Agency for Healthcare Research and Quality is one of twelve agencies within the United States Department of Health and Human Services (HHS). The agency is headquartered in North Bethesda, Maryland, a suburb of Washington, D.C.. It was established as the Agency for Health Care Policy and Research (AHCPR) in 1989 as a constituent unit of the Public Health Service (PHS) to enhance the quality, appropriateness, and effectiveness of health care services and access to care by conducting and supporting research, demonstration projects, and evaluations; developing guidelines; and disseminating information on health care services and delivery systems.
The pneumonia severity index (PSI) or PORT Score is a clinical prediction rule that medical practitioners can use to calculate the probability of morbidity and mortality among patients with community acquired pneumonia.
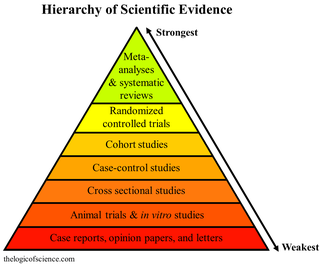
A systematic review is a scholarly synthesis of the evidence on a clearly presented topic using critical methods to identify, define and assess research on the topic. A systematic review extracts and interprets data from published studies on the topic, then analyzes, describes, critically appraises and summarizes interpretations into a refined evidence-based conclusion. For example, a systematic review of randomized controlled trials is a way of summarizing and implementing evidence-based medicine.
eMedicine is an online clinical medical knowledge base founded in 1996 by doctors Scott Plantz and Jonathan Adler, and computer engineer Jeffrey Berezin. The eMedicine website consists of approximately 6,800 medical topic review articles, each of which is associated with a clinical subspecialty "textbook". The knowledge base includes over 25,000 clinically multimedia files.
The American College of Radiology (ACR), founded in 1923, is a professional medical society representing nearly 40,000 diagnostic radiologists, radiation oncologists, interventional radiologists, nuclear medicine physicians and medical physicists.
Patient safety is a discipline that emphasizes safety in health care through the prevention, reduction, reporting and analysis of error and other types of unnecessary harm that often lead to adverse patient events. The frequency and magnitude of avoidable adverse events, often known as patient safety incidents, experienced by patients was not well known until the 1990s, when multiple countries reported significant numbers of patients harmed and killed by medical errors. Recognizing that healthcare errors impact 1 in every 10 patients around the world, the World Health Organization (WHO) calls patient safety an endemic concern. Indeed, patient safety has emerged as a distinct healthcare discipline supported by an immature yet developing scientific framework. There is a significant transdisciplinary body of theoretical and research literature that informs the science of patient safety with mobile health apps being a growing area of research.
A patient safety organization (PSO) is a group, institution, or association that improves medical care by reducing medical errors. Common functions of patient safety organizations are data collection, analysis, reporting, education, funding, and advocacy. A PSO differs from a Federally designed Patient Safety Organization (PSO), which provides health care providers in the U.S. privilege and confidentiality protections for efforts to improve patient safety and the quality of patient care delivery
The Guidelines International Network (GIN) is an international scientific association of organisations and individuals interested and involved in development and application of evidence-based guidelines and health care information. The network supports evidence-based health care and improved health outcomes by reducing inappropriate variation throughout the world.
Health services research (HSR) became a burgeoning field in North America in the 1960s, when scientific information and policy deliberation began to coalesce. Sometimes also referred to as health systems research or health policy and systems research (HPSR), HSR is a multidisciplinary scientific field that examines how people get access to health care practitioners and health care services, how much care costs, and what happens to patients as a result of this care. HSR utilizes all qualitative and quantitative methods across the board to ask questions of the healthcare system. It focuses on performance, quality, effectiveness and efficiency of health care services as they relate to health problems of individuals and populations, as well as health care systems and addresses wide-ranging topics of structure, processes, and organization of health care services; their use and people's access to services; efficiency and effectiveness of health care services; the quality of healthcare services and its relationship to health status, and; the uses of medical knowledge.

The German Agency for Quality in Medicine (AEZQ) - in German "Ärztliches Zentrum für Qualität in der Medizin (ÄZQ)", established in 1995 and located in Berlin, co-ordinates healthcare quality programmes with special focus on evidence-based medicine, medical guidelines, patient empowerment, patient safety programs, and quality management.

ECRI is an independent nonprofit organization tasked with "improving the safety, quality, and cost-effectiveness of care across all healthcare settings worldwide."

The Healthcare Cost and Utilization Project is a family of healthcare databases and related software tools and products from the United States that is developed through a Federal-State-Industry partnership and sponsored by the Agency for Healthcare Research and Quality (AHRQ).
Decision aids are interventions or tools designed to facilitate shared decision making and patient participation in health care decisions.

The National Collaborating Centre for Mental Health (NCCMH) is a collaboration between the Royal College of Psychiatrists and the Centre for Outcomes Research and Effectiveness at University College London (UCL). The NCCMH aims to promote the role of evidence synthesis in making informed judgments about healthcare policy. The NCCMH has a history of developing guidelines, conducting systematic reviews and developing implementation guidance for commissioners and service providers. Formed in 2001, on 1 April 2016 a new guideline development centre, the National Guideline Alliance, based at the Royal College of Obstetricians and Gynaecologists took over the clinical guideline programme that had been run by NCCMH.
The Improvement Science Research Network (ISRN) is a research network for academics and physicians who are conducting studies in the new medical field of improvement science.
Health care quality is a level of value provided by any health care resource, as determined by some measurement. As with quality in other fields, it is an assessment of whether something is good enough and whether it is suitable for its purpose. The goal of health care is to provide medical resources of high quality to all who need them; that is, to ensure good quality of life, cure illnesses when possible, to extend life expectancy, and so on. Researchers use a variety of quality measures to attempt to determine health care quality, including counts of a therapy's reduction or lessening of diseases identified by medical diagnosis, a decrease in the number of risk factors which people have following preventive care, or a survey of health indicators in a population who are accessing certain kinds of care.
Guidances for statistics in regulatory affairs refers to specific documents or guidelines that provide instructions, recommendations, and standards pertaining to the application of statistical methodologies and practices within the regulatory framework of industries such as pharmaceuticals and medical devices. These guidances serve as a reference for statisticians, researchers, and professionals involved in designing, conducting, analyzing, and reporting studies and trials in compliance with regulatory requirements. These documents embody the prevailing perspectives of regulatory agencies on specific subjects. It is worth noting that in the United States, the term "Guidances" is used, while in Europe, the term "Guidelines" is employed.







