
Biogas is a mixture of gases, primarily consisting of methane, carbon dioxide and hydrogen sulphide, produced from raw materials such as agricultural waste, manure, municipal waste, plant material, sewage, green waste, wastewater, and food waste. It is a renewable energy source.

Ground-level ozone (O3), also known as surface-level ozone and tropospheric ozone, is a trace gas in the troposphere (the lowest level of the Earth's atmosphere), with an average concentration of 20–30 parts per billion by volume (ppbv), with close to 100 ppbv in polluted areas. Ozone is also an important constituent of the stratosphere, where the ozone layer (2 to 8 parts per million ozone) exists which is located between 10 and 50 kilometers above the Earth's surface. The troposphere extends from the ground up to a variable height of approximately 14 kilometers above sea level. Ozone is least concentrated in the ground layer (or planetary boundary layer) of the troposphere. Ground-level or tropospheric ozone is created by chemical reactions between NOx gases (oxides of nitrogen produced by combustion) and volatile organic compounds (VOCs). The combination of these chemicals in the presence of sunlight form ozone. Its concentration increases as height above sea level increases, with a maximum concentration at the tropopause. About 90% of total ozone in the atmosphere is in the stratosphere, and 10% is in the troposphere. Although tropospheric ozone is less concentrated than stratospheric ozone, it is of concern because of its health effects. Ozone in the troposphere is considered a greenhouse gas, and may contribute to global warming.

The pyrolysis process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements pyro "fire", "heat", "fever" and lysis "separating".

Gasification is a process that converts biomass- or fossil fuel-based carbonaceous materials into gases, including as the largest fractions: nitrogen (N2), carbon monoxide (CO), hydrogen (H2), and carbon dioxide (CO2). This is achieved by reacting the feedstock material at high temperatures (typically >700 °C), without combustion, via controlling the amount of oxygen and/or steam present in the reaction. The resulting gas mixture is called syngas (from synthesis gas) or producer gas and is itself a fuel due to the flammability of the H2 and CO of which the gas is largely composed. Power can be derived from the subsequent combustion of the resultant gas, and is considered to be a source of renewable energy if the gasified compounds were obtained from biomass feedstock.

Alternative fuels, also known as non-conventional and advanced fuels, are fuels derived from sources other than fossil fuels. Some well-known alternative fuels include hydrogen, bio-diesel, bio-alcohol, refuse-derived fuel, chemically stored electricity, non-fossil methane, non-fossil natural gas, vegetable oil, propane and other biomass sources.
Eastman Chemical Company is an American company primarily involved in the chemical industry. Once a subsidiary of Kodak, today it is an independent global specialty materials company that produces a broad range of advanced materials, chemicals and fibers for everyday purposes. Founded in 1920 and based in Kingsport, Tennessee, the company now has more than 50 manufacturing sites worldwide and employs approximately 14,000 people.

A gas stove is a stove that is fuelled by combustible gas such as syngas, natural gas, propane, butane, liquefied petroleum gas or other flammable gas. Before the advent of gas, cooking stoves relied on solid fuels such as coal or wood. The first gas stoves were developed in the 1820s and a gas stove factory was established in England in 1836. This new cooking technology had the advantage of being easily adjustable and could be turned off when not in use. The gas stove, however, did not become a commercial success until the 1880s, by which time supplies of piped gas were available in cities and large towns in Britain. The stoves became widespread on the European Continent and in the United States in the early 20th century.

Landfill gas is a mix of different gases created by the action of microorganisms within a landfill as they decompose organic waste, including for example, food waste and paper waste. Landfill gas is approximately forty to sixty percent methane, with the remainder being mostly carbon dioxide. Trace amounts of other volatile organic compounds (VOCs) comprise the remainder (<1%). These trace gases include a large array of species, mainly simple hydrocarbons.

Industrial gases are the gaseous materials that are manufactured for use in industry. The principal gases provided are nitrogen, oxygen, carbon dioxide, argon, hydrogen, helium and acetylene, although many other gases and mixtures are also available in gas cylinders. The industry producing these gases is also known as industrial gas, which is seen as also encompassing the supply of equipment and technology to produce and use the gases. Their production is a part of the wider chemical Industry.
Enhanced oil recovery, also called tertiary recovery, is the extraction of crude oil from an oil field that cannot be extracted otherwise. EOR can extract 30% to 60% or more of a reservoir's oil, compared to 20% to 40% using primary and secondary recovery. According to the US Department of Energy, carbon dioxide and water are injected along with one of three EOR techniques: thermal injection, gas injection, and chemical injection. More advanced, speculative EOR techniques are sometimes called quaternary recovery.

Waste-to-energy (WtE) or energy-from-waste (EfW) is the process of generating energy in the form of electricity and/or heat from the primary treatment of waste, or the processing of waste into a fuel source. WtE is a form of energy recovery. Most WtE processes generate electricity and/or heat directly through combustion, or produce a combustible fuel commodity, such as methane, methanol, ethanol or synthetic fuels.

Biodegradable plastics are plastics that can be decomposed by the action of living organisms, usually microbes, into water, carbon dioxide, and biomass. Biodegradable plastics are commonly produced with renewable raw materials, micro-organisms, petrochemicals, or combinations of all three.

Greenhouse gas emissions from human activities strengthen the greenhouse effect, contributing to climate change. Most is carbon dioxide from burning fossil fuels: coal, oil, and natural gas. The largest emitters include coal in China and large oil and gas companies. Human-caused emissions have increased atmospheric carbon dioxide by about 50% over pre-industrial levels. The growing levels of emissions have varied, but have been consistent among all greenhouse gases (GHGs). Emissions in the 2010s averaged 56 billion tons a year, higher than any decade before.

The United States produced 5.2 billion metric tons of carbon dioxide equivalent greenhouse gas (GHG) emissions in 2020, the second largest in the world after greenhouse gas emissions by China and among the countries with the highest greenhouse gas emissions per person. In 2019 China is estimated to have emitted 27% of world GHG, followed by the United States with 11%, then India with 6.6%. In total the United States has emitted a quarter of world GHG, more than any other country. Annual emissions are over 15 tons per person and, amongst the top eight emitters, is the highest country by greenhouse gas emissions per person. However, the IEA estimates that the richest decile in the US emits over 55 tonnes of CO2 per capita each year. Because coal-fired power stations are gradually shutting down, in the 2010s emissions from electricity generation fell to second place behind transportation which is now the largest single source. In 2020, 27% of the GHG emissions of the United States were from transportation, 25% from electricity, 24% from industry, 13% from commercial and residential buildings and 11% from agriculture. In 2021, the electric power sector was the second largest source of U.S. greenhouse gas emissions, accounting for 25% of the U.S. total. These greenhouse gas emissions are contributing to climate change in the United States, as well as worldwide.

Upcycling, also known as creative reuse, is the process of transforming by-products, waste materials, useless, or unwanted products into new materials or products perceived to be of greater quality, such as artistic value or environmental value.

Republic Services is an American waste disposal company whose services include non-hazardous solid waste collection, waste transfer, waste disposal, recycling, and energy services. It is the second largest provider of waste disposal in the United States after Waste Management Corporation.

Methane is a chemical compound with the chemical formula CH4. It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Earth makes it an economically attractive fuel, although capturing and storing it poses technical challenges due to its gaseous state under normal conditions for temperature and pressure.
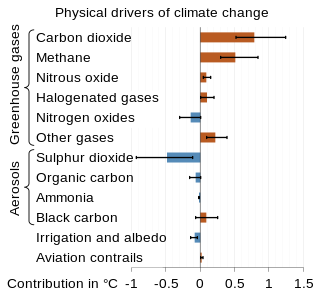
Atmospheric methane is the methane present in Earth's atmosphere. The concentration of atmospheric methane—one of the most potent greenhouse gases—is increasing due to methane emissions, and is causing climate change.

Rocket Lab USA, Inc. is a publicly traded aerospace manufacturer and launch service provider. The company operates lightweight Electron orbital rockets, which provide dedicated launches for small satellites. Rocket Lab also plans to build a larger Neutron rocket as early as 2024. Electron rockets have launched 33 times from either Rocket Lab's Launch Complex 1 in New Zealand or the Mid-Atlantic Regional Spaceport (MARS) in the United States. Two attempts have been made at recovery of the Electron booster by helicopter. In addition, 4 attempts have been made at soft water recovery. As of 2022, Rocket Lab is developing the bigger Neutron reusable unibody rocket; Photon satellite buses; and Rutherford, Curie, HyperCurie, and Archimedes rocket engines.
Calysta is a multinational biotechnology firm based in San Mateo, California. The company develops industrial processes that utilize microorganisms to convert methane into protein for seafood, livestock feed and other food ingredients. It operates a demonstration plant in Teesside, England, that uses methanotroph bacteria to convert methane into single cell protein currently approved for use in fish and livestock feed in the European Union. The firm is a spinout of DNA 2.0, the largest US-based provider of synthetic genes for industrial and academic use.
















