Related Research Articles

Adenosine triphosphate (ATP) is a nucleoside triphosphate that provides energy to drive and support many processes in living cells, such as muscle contraction, nerve impulse propagation, and chemical synthesis. Found in all known forms of life, it is often referred to as the "molecular unit of currency" for intracellular energy transfer.

A tyrosine kinase is an enzyme that can transfer a phosphate group from ATP to the tyrosine residues of specific proteins inside a cell. It functions as an "on" or "off" switch in many cellular functions.

In chemistry, pyrophosphates are phosphorus oxyanions that contain two phosphorus atoms in a P−O−P linkage. A number of pyrophosphate salts exist, such as disodium pyrophosphate and tetrasodium pyrophosphate, among others. Often pyrophosphates are called diphosphates. The parent pyrophosphates are derived from partial or complete neutralization of pyrophosphoric acid. The pyrophosphate bond is also sometimes referred to as a phosphoanhydride bond, a naming convention which emphasizes the loss of water that occurs when two phosphates form a new P−O−P bond, and which mirrors the nomenclature for anhydrides of carboxylic acids. Pyrophosphates are found in ATP and other nucleotide triphosphates, which are important in biochemistry. The term pyrophosphate is also the name of esters formed by the condensation of a phosphorylated biological compound with inorganic phosphate, as for dimethylallyl pyrophosphate. This bond is also referred to as a high-energy phosphate bond.

Biochemistry is the study of the chemical processes in living organisms. It deals with the structure and function of cellular components such as proteins, carbohydrates, lipids, nucleic acids and other biomolecules.
The iron–sulfur world hypothesis is a set of proposals for the origin of life and the early evolution of life advanced in a series of articles between 1988 and 1992 by Günter Wächtershäuser, a Munich patent lawyer with a degree in chemistry, who had been encouraged and supported by philosopher Karl R. Popper to publish his ideas. The hypothesis proposes that early life may have formed on the surface of iron sulfide minerals, hence the name. It was developed by retrodiction from extant biochemistry in conjunction with chemical experiments.

Nucleoside-diphosphate kinases are enzymes that catalyze the exchange of terminal phosphate between different nucleoside diphosphates (NDP) and triphosphates (NTP) in a reversible manner to produce nucleotide triphosphates. Many NDP serve as acceptor while NTP are donors of phosphate group. The general reaction via ping-pong mechanism is as follows: XDP + YTP ←→ XTP + YDP. NDPK activities maintain an equilibrium between the concentrations of different nucleoside triphosphates such as, for example, when guanosine triphosphate (GTP) produced in the citric acid (Krebs) cycle is converted to adenosine triphosphate (ATP). Other activities include cell proliferation, differentiation and development, signal transduction, G protein-coupled receptor, endocytosis, and gene expression.

Phosphatidylinositol (3,4)-bisphosphate is a minor phospholipid component of cell membranes, yet an important second messenger. The generation of PtdIns(3,4)P2 at the plasma membrane activates a number of important cell signaling pathways.
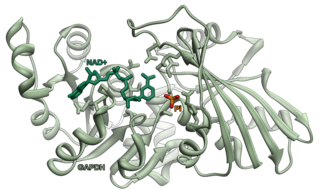
Glyceraldehyde 3-phosphate dehydrogenase is an enzyme of about 37kDa that catalyzes the sixth step of glycolysis and thus serves to break down glucose for energy and carbon molecules. In addition to this long established metabolic function, GAPDH has recently been implicated in several non-metabolic processes, including transcription activation, initiation of apoptosis, ER-to-Golgi vesicle shuttling, and fast axonal, or axoplasmic transport. In sperm, a testis-specific isoenzyme GAPDHS is expressed.
GRE Subject Biochemistry, Cell and Molecular Biology was a standardized exam provided by ETS that was discontinued in December 2016. It is a paper-based exam and there are no computer-based versions of it. ETS places this exam three times per year: once in April, once in October and once in November. Some graduate programs in the United States recommend taking this exam, while others require this exam score as a part of the application to their graduate programs. ETS sends a bulletin with a sample practice test to each candidate after registration for the exam. There are 180 questions within the biochemistry subject test.
Chemical modification refers to a number of various processes involving the alteration of the chemical constitution or structure of molecules.

Diphosphomevalonate decarboxylase (EC 4.1.1.33), most commonly referred to in scientific literature as mevalonate diphosphate decarboxylase, is an enzyme that catalyzes the chemical reaction
Exopolyphosphatase (PPX) is a phosphatase enzyme which catalyzes the hydrolysis of inorganic polyphosphate, a linear molecule composed of up to 1000 or more monomers linked by phospho-anhydride bonds. PPX is a processive exophosphatase, which means that it begins at the ends of the polyphosphate chain and cleaves the phospho-anhydride bonds to release orthophosphate as it moves along the polyphosphate molecule. PPX has several characteristics which distinguish it from other known polyphosphatases, namely that it does not act on ATP, has a strong preference for long chain polyphosphate, and has a very low affinity for polyphosphate molecules with less than 15 phosphate monomers.

The enzyme phosphatidate phosphatase (PAP, EC 3.1.3.4) is a key regulatory enzyme in lipid metabolism, catalyzing the conversion of phosphatidate to diacylglycerol:
The following outline is provided as an overview of and topical guide to cell biology:
In enzymology, a polyphosphate kinase, or polyphosphate polymerase, is an enzyme that catalyzes the formation of polyphosphate from ATP, with chain lengths of up to a thousand or more orthophosphate moieties.
A protocell is a self-organized, endogenously ordered, spherical collection of lipids proposed as a rudimentary precursor to cells during the origin of life. A central question in evolution is how simple protocells first arose and how their progeny could diversify, thus enabling the accumulation of novel biological emergences over time. Although a functional protocell has not yet been achieved in a laboratory setting, the goal to understand the process appears well within reach.
Src kinase family is a family of non-receptor tyrosine kinases that includes nine members: Src, Yes, Fyn, and Fgr, forming the SrcA subfamily, Lck, Hck, Blk, and Lyn in the SrcB subfamily, and Frk in its own subfamily. Frk has homologs in invertebrates such as flies and worms, and Src homologs exist in organisms as diverse as unicellular choanoflagellates, but the SrcA and SrcB subfamilies are specific to vertebrates. Src family kinases contain six conserved domains: a N-terminal myristoylated segment, a SH2 domain, a SH3 domain, a linker region, a tyrosine kinase domain, and C-terminal tail.
A scenario is a set of related concepts pertinent to the origin of life (abiogenesis), such as the iron-sulfur world. Many alternative abiogenesis scenarios have been proposed by scientists in a variety of fields from the 1950s onwards in an attempt to explain how the complex mechanisms of life could have come into existence. These include hypothesized ancient environments that might have been favourable for the origin of life, and possible biochemical mechanisms.
This glossary of cellular and molecular biology is a list of definitions of terms and concepts commonly used in the study of cell biology, molecular biology, and related disciplines, including molecular genetics, biochemistry, and microbiology. It is split across two articles:
This glossary of cellular and molecular biology is a list of definitions of terms and concepts commonly used in the study of cell biology, molecular biology, and related disciplines, including genetics, biochemistry, and microbiology. It is split across two articles:
References
- 1 2 3 4 5 6 7 8 Cavalier-Smith T (2001). "Obcells as proto-organisms: membrane heredity, lithophosphorylation, and the origins of the genetic code, the first cells, and photosynthesis". J. Mol. Evol. 53 (4–5): 555–95. Bibcode:2001JMolE..53..555C. CiteSeerX 10.1.1.607.8378 . doi:10.1007/s002390010245. PMID 11675615. S2CID 21832452.
- 1 2 Deamer, David (June 1997). "The First Living Systems: a Bioenergetic Perspective". Microbiology and Molecular Biology Reviews. 61 (2): 239–61. doi:10.1128/mmbr.61.2.239-261.1997. PMC 232609 . PMID 9184012.
- ↑ Kornberg, A (1999). "Inorganic Polyphosphate: a Molecule of Many Functions". Annual Review of Biochemistry. 68: 89–125. doi:10.1146/annurev.biochem.68.1.89. PMID 10872445.
- ↑ Hsieh, P (March 1996). "Cloning, Expression, and Characterization of Polyphosphate Glucokinase from Mycobacterium Tuberculosis". National Center of Biotechnology Information.
- ↑ Levy, Matthew (July 7, 1998). "The Stability of the RNA Bases: Implications for the Origin of Life". Proceedings of the National Academy of Sciences of the United States of America. 95 (14): 7933–7938. Bibcode:1998PNAS...95.7933L. doi: 10.1073/pnas.95.14.7933 . PMC 20907 . PMID 9653118.