
The Office of Rare Diseases Research is a division of the US National Center for Advancing Translational Sciences (NCATS) that oversees the Rare Diseases Clinical Research Network and Genetic and Rare Diseases Information Center. [1]

The Office of Rare Diseases Research is a division of the US National Center for Advancing Translational Sciences (NCATS) that oversees the Rare Diseases Clinical Research Network and Genetic and Rare Diseases Information Center. [1]
The Office of Rare Diseases Research was established in 1993 within the Office of the Director of the NIH. Its responsibilities were mandated by statute by the Rare Diseases Act of 2002. [2] [3] In 2011, the office became part of the newly created NCATS. [4] ORDR is currently headed by Dr. Anne R. Pariser, who took over the position in February 2018. [5]

The National Institutes of Health, commonly referred to as NIH, is the primary agency of the United States government responsible for biomedical and public health research. It was founded in the late 1880s and is now part of the United States Department of Health and Human Services. Many NIH facilities are located in Bethesda, Maryland, and other nearby suburbs of the Washington metropolitan area, with other primary facilities in the Research Triangle Park in North Carolina and smaller satellite facilities located around the United States. The NIH conducts its own scientific research through the NIH Intramural Research Program (IRP) and provides major biomedical research funding to non-NIH research facilities through its Extramural Research Program.
A rare disease is a disease that affects a small percentage of the population. In some parts of the world, an orphan disease is a rare disease whose rarity means there is a lack of a market large enough to gain support and resources for discovering treatments for it, except by the government granting economically advantageous conditions to creating and selling such treatments. Orphan drugs are ones so created or sold.
The National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) is one of the institutes and centers that make up the National Institutes of Health, an agency of the United States Department of Health and Human Services (HHS).
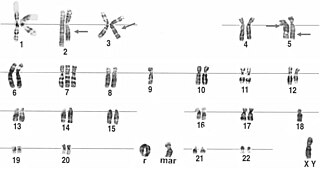
A ring chromosome is an aberrant chromosome whose ends have fused together to form a ring. Ring chromosomes were first discovered by Lilian Vaughan Morgan in 1926. A ring chromosome is denoted by the symbol r in human genetics and R in Drosophila genetics. Ring chromosomes may form in cells following genetic damage by mutagens like radiation, but they may also arise spontaneously during development.

Köhler disease is a rare bone disorder of the foot found in children between six and nine years of age. The disease typically affects boys, but it can also affect girls. It was first described in 1908 by Alban Köhler (1874–1947), a German radiologist. Dr. A. Köhler noted that children with foot pain displayed characteristics, within their x-rays, of irregularity in growth and development of the tarsal navicular bone in the foot. Furthermore, Köhler disease is known to affect five times more boys than girls and typically, only one foot is affected. The disease was then found to belong to a group of conditions called osteochondroses, which disturb bone growth at ossification centres which occurs during bone development.

Heřmanský–Pudlák syndrome is an extremely rare autosomal recessive disorder which results in oculocutaneous albinism, bleeding problems due to a platelet abnormality, and storage of an abnormal fat-protein compound. It is thought to affect around 1 in 500,000 people worldwide, with a significantly higher occurrence in Puerto Ricans, with a prevalence of 1 in 1800. Many of the clinical research studies on the disease have been conducted in Puerto Rico.
Clear-cell carcinoma, also known as clear-cell adenocarcinoma and mesonephroma, is an epithelial-cell-derived carcinoma characterized by the presence of clear cells observed during histological, diagnostic assessment. This form of cancer is classified as a rare cancer with an incidence of 4.8% in white patients, 3.1% in black patients, and 11.1% in Asian patients.
The National Center for Research Resources (NCRR) was a center within the National Institutes of Health, a United States government agency. NCRR provided funding to laboratory scientists and researchers for facilities and tools in the goal of curing and treating diseases.

Translational research is research aimed at translating (converting) results in basic research into results that directly benefit humans. The term is used in science and technology, especially in biology and medical science. As such, translational research forms a subset of applied research.
The National Health Council (NHC) is a nonprofit association of health organizations.

The Vaccine Research Center (VRC), is an intramural division of the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), US Department of Health and Human Services (HHS). The mission of the VRC is to discover and develop both vaccines and antibody-based products that target infectious diseases.
The National Center for Advancing Translational Sciences (NCATS) was established on December 23, 2011 and is located in Bethesda, Maryland. NCATS is one of 27 institutes and centers of the U.S. National Institutes of Health (NIH), an agency of the U.S. Department of Health and Human Services. The mission of NCATS is to transform scientific discoveries into new treatments and cures for disease that can be delivered faster to patients. The budget provided to NCATS for fiscal year 2018 is $557,373,000.
The Rare Diseases Clinical Research Network (RDCRN) is an initiative of the US Office of Rare Diseases Research (ORDR). RDCRN is funded by the ORDR, the National Center for Advancing Translational Sciences and collaborating institute centers. The RDCRN is designed to advance medical research on rare diseases by providing support for clinical studies and facilitating collaboration, study enrollment and data sharing. Through the RDCRN consortia, physician scientists and their multidisciplinary teams work together with patient advocacy groups to study more than 200 rare diseases at sites across the nation.
The Rare Diseases Clinical Research Network (RDCRN) Contact Registry is a patient contact registry sponsored by the National Institutes of Health (NIH). The RDCRN Contact Registry collects and stores the contact information of people who want to participate in RDCRN-sponsored research or learn more about RDCRN research. It connects patients with researchers in order to advance rare diseases research.

The Rare Disease Act of 2002 is a law passed in the United States that establishes the statutory authorization for the Office of Rare Diseases as a federal entity able to recommend a national research agenda, coordinate research, and provide educational activities for researchers.
Dr. Vinod Scaria FRSB, FRSPH is an Indian biologist, medical researcher pioneering in Precision Medicine and Clinical Genomics in India. He is best known for sequencing the first Indian genome. He was also instrumental in the sequencing of The first Sri Lankan Genome, analysis of the first Malaysian Genome sequencing and analysis of the Wild-type strain of Zebrafish and the IndiGen programme on Genomics for Public Health in India

Marshall L. Summar is a physician, clinical geneticist and academic specializing in the field of genetics and rare disease.

Joni L. Rutter is an American geneticist and director of the National Center for Advancing Translational Sciences (NCATS). Rutter was previously director of the scientific programs in the All of Us initiative and served as the neuroscience and behavior division director at the National Institute on Drug Abuse. Her scientific experience includes clinical research in human genetics and environmental risk factors focusing on the fields of cancer and addiction.

Kathy Lynn Hudson is an American microbiologist specializing in science policy. She was the deputy director for science, outreach, and policy at the National Institutes of Health from October 2010 to January 2017. Hudson assisted in the creation and launch of All of Us, the BRAIN initiative, and the National Center for Advancing Translational Sciences. She founded the Genetics and Public Policy Center at Johns Hopkins University in 2002. Hudson is an advocate for women in science.