Related Research Articles

Spectroscopy is the field of study that measures and interprets electromagnetic spectra. In narrower contexts, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum.
Atomic, molecular, and optical physics (AMO) is the study of matter–matter and light–matter interactions, at the scale of one or a few atoms and energy scales around several electron volts. The three areas are closely interrelated. AMO theory includes classical, semi-classical and quantum treatments. Typically, the theory and applications of emission, absorption, scattering of electromagnetic radiation (light) from excited atoms and molecules, analysis of spectroscopy, generation of lasers and masers, and the optical properties of matter in general, fall into these categories.

Inductively coupled plasma mass spectrometry (ICP-MS) is a type of mass spectrometry that uses an inductively coupled plasma to ionize the sample. It atomizes the sample and creates atomic and small polyatomic ions, which are then detected. It is known and used for its ability to detect metals and several non-metals in liquid samples at very low concentrations. It can detect different isotopes of the same element, which makes it a versatile tool in isotopic labeling.

Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule is called an ion. Ionization can result from the loss of an electron after collisions with subatomic particles, collisions with other atoms, molecules and ions, or through the interaction with electromagnetic radiation. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.

In astronomy, the interstellar medium (ISM) is the matter and radiation that exists in the space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, as well as dust and cosmic rays. It fills interstellar space and blends smoothly into the surrounding intergalactic space. The energy that occupies the same volume, in the form of electromagnetic radiation, is the interstellar radiation field. Although the density of atoms in the ISM is usually far below that in the best laboratory vacuums, the mean free path between collisions is short compared to typical interstellar lengths, so on these scales the ISM behaves as a gas (more precisely, as a plasma: it is everywhere at least slightly ionized), responding to pressure forces, and not as a collection of non-interacting particles.
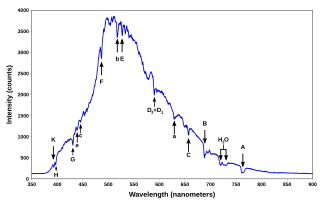
A spectral line is a weaker or stronger region in an otherwise uniform and continuous spectrum. It may result from emission or absorption of light in a narrow frequency range, compared with the nearby frequencies. Spectral lines are often used to identify atoms and molecules. These "fingerprints" can be compared to the previously collected ones of atoms and molecules, and are thus used to identify the atomic and molecular components of stars and planets, which would otherwise be impossible.

An ion source is a device that creates atomic and molecular ions. Ion sources are used to form ions for mass spectrometers, optical emission spectrometers, particle accelerators, ion implanters and ion engines.
Plasma diagnostics are a pool of methods, instruments, and experimental techniques used to measure properties of a plasma, such as plasma components' density, distribution function over energy (temperature), their spatial profiles and dynamics, which enable to derive plasma parameters.
In physics, atomic spectroscopy is the study of the electromagnetic radiation absorbed and emitted by atoms. Since unique elements have unique emission spectra, atomic spectroscopy is applied for determination of elemental compositions. It can be divided by atomization source or by the type of spectroscopy used. In the latter case, the main division is between optical and mass spectrometry. Mass spectrometry generally gives significantly better analytical performance, but is also significantly more complex. This complexity translates into higher purchase costs, higher operational costs, more operator training, and a greater number of components that can potentially fail. Because optical spectroscopy is often less expensive and has performance adequate for many tasks, it is far more common. Atomic absorption spectrometers are one of the most commonly sold and used analytical devices.

A glow discharge is a plasma formed by the passage of electric current through a gas. It is often created by applying a voltage between two electrodes in a glass tube containing a low-pressure gas. When the voltage exceeds a value called the striking voltage, the gas ionization becomes self-sustaining, and the tube glows with a colored light. The color depends on the gas used.
Penning ionization is a form of chemi-ionization, an ionization process involving reactions between neutral atoms or molecules. The Penning effect is put to practical use in applications such as gas-discharge neon lamps and fluorescent lamps, where the lamp is filled with a Penning mixture to improve the electrical characteristics of the lamps.
High-harmonic generation (HHG) is a non-linear process during which a target is illuminated by an intense laser pulse. Under such conditions, the sample will emit the high harmonics of the generation beam. Due to the coherent nature of the process, high-harmonics generation is a prerequisite of attosecond physics.

Atomic emission spectroscopy (AES) is a method of chemical analysis that uses the intensity of light emitted from a flame, plasma, arc, or spark at a particular wavelength to determine the quantity of an element in a sample. The wavelength of the atomic spectral line in the emission spectrum gives the identity of the element while the intensity of the emitted light is proportional to the number of atoms of the element. The sample may be excited by various methods.

Plasma is one of four fundamental states of matter characterized by the presence of a significant portion of charged particles in any combination of ions or electrons. It is the most abundant form of ordinary matter in the universe, mostly in stars, but also dominating the rarefied intracluster medium and intergalactic medium. Plasma can be artificially generated, for example, by heating a neutral gas or subjecting it to a strong electromagnetic field.
Quantemol Ltd is based in University College London initiated by Professor Jonathan Tennyson FRS and Dr. Daniel Brown in 2004. The company initially developed a unique software tool, Quantemol-N, which provides full accessibility to the highly sophisticated UK molecular R-matrix codes, used to model electron polyatomic molecule interactions. Since then Quantemol has widened to further types of simulation, with plasmas and industrial plasma tools, in Quantemol-VT in 2013 and launched in 2016 a sustainable database Quantemol-DB, representing the chemical and radiative transport properties of a wide range of plasmas.
An excimer lamp is a source of ultraviolet light based on spontaneous emission of excimer (exciplex) molecules.
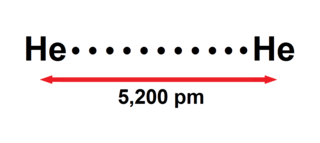
The helium dimer is a van der Waals molecule with formula He2 consisting of two helium atoms. This chemical is the largest diatomic molecule—a molecule consisting of two atoms bonded together. The bond that holds this dimer together is so weak that it will break if the molecule rotates, or vibrates too much. It can only exist at very low cryogenic temperatures.
Argon compounds, the chemical compounds that contain the element argon, are rarely encountered due to the inertness of the argon atom. However, compounds of argon have been detected in inert gas matrix isolation, cold gases, and plasmas, and molecular ions containing argon have been made and also detected in space. One solid interstitial compound of argon, Ar1C60 is stable at room temperature. Ar1C60 was discovered by the CSIRO.
The magnesium argide ion, MgAr+ is an ion composed of one ionised magnesium atom, Mg+ and an argon atom. It is important in inductively coupled plasma mass spectrometry and in the study of the field around the magnesium ion. The ionization potential of magnesium is lower than the first excitation state of argon, so the positive charge in MgAr+ will reside on the magnesium atom. Neutral MgAr molecules can also exist in an excited state.

Resonance ionization is a process in optical physics used to excite a specific atom beyond its ionization potential to form an ion using a beam of photons irradiated from a pulsed laser light. In resonance ionization, the absorption or emission properties of the emitted photons are not considered, rather only the resulting excited ions are mass-selected, detected and measured. Depending on the laser light source used, one electron can be removed from each atom so that resonance ionization produces an efficient selectivity in two ways: elemental selectivity in ionization and isotopic selectivity in measurement.
References
- ↑ Pfaff, J.; Begemann, M. H.; Saykally, R. J. (1984). "An investigation of the laser optogalvanic effect for atoms and molecules in recombination-limited plasmas" (PDF). Molecular Physics. 52 (3): 541–566. Bibcode:1984MolPh..52..541P. doi:10.1080/00268978400101391.
- ↑ Ochkin, V. N.; Preobrazhenskii, N. G.; Sobolev, N. N.; Shaparev, N. Ya. (1986). "Optogalvanic effect in plasmas and gases". Sov. Phys. Usp. 29 (3): 260. Bibcode:1986SvPhU..29..260O. doi:10.1070/PU1986v029n03ABEH003187.
- ↑ Reddy. "Laser Optogalvanic Spectroscopy: Experimental Details and Potential Applications in R&D" (PDF).