Related Research Articles

Behavioral neuroscience, also known as biological psychology, biopsychology, or psychobiology, is the application of the principles of biology to the study of physiological, genetic, and developmental mechanisms of behavior in humans and other animals.

Melanopsin is a type of photopigment belonging to a larger family of light-sensitive retinal proteins called opsins and encoded by the gene Opn4. In the mammalian retina, there are two additional categories of opsins, both involved in the formation of visual images: rhodopsin and photopsin in the rod and cone photoreceptor cells, respectively.
Channelrhodopsins are a subfamily of retinylidene proteins (rhodopsins) that function as light-gated ion channels. They serve as sensory photoreceptors in unicellular green algae, controlling phototaxis: movement in response to light. Expressed in cells of other organisms, they enable light to control electrical excitability, intracellular acidity, calcium influx, and other cellular processes. Channelrhodopsin-1 (ChR1) and Channelrhodopsin-2 (ChR2) from the model organism Chlamydomonas reinhardtii are the first discovered channelrhodopsins. Variants that are sensitive to different colors of light or selective for specific ions have been cloned from other species of algae and protists.

Photostimulation is the use of light to artificially activate biological compounds, cells, tissues, or even whole organisms. Photostimulation can be used to noninvasively probe various relationships between different biological processes, using only light. In the long run, photostimulation has the potential for use in different types of therapy, such as migraine headache. Additionally, photostimulation may be used for the mapping of neuronal connections between different areas of the brain by “uncaging” signaling biomolecules with light. Therapy with photostimulation has been called light therapy, phototherapy, or photobiomodulation.
A receptor activated solely by a synthetic ligand (RASSL) or designer receptor exclusively activated by designer drugs (DREADD), is a class of artificially engineered protein receptors used in the field of chemogenetics which are selectively activated by certain ligands. They are used in biomedical research, in particular in neuroscience to manipulate the activity of neurons.
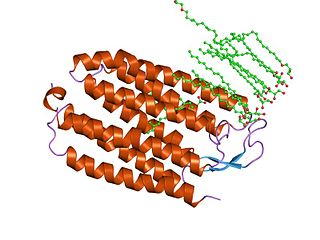
Halorhodopsin is a seven-transmembrane retinylidene protein from microbial rhodopsin family. It is a chloride-specific light-activated ion pump found in archaea known as halobacteria. It is activated by green light wavelengths of approximately 578nm. Halorhodopsin also shares sequence similarity to channelrhodopsin, a light-gated ion channel.
Light-gated ion channels are a family of ion channels regulated by electromagnetic radiation. Other gating mechanisms for ion channels include voltage-gated ion channels, ligand-gated ion channels, mechanosensitive ion channels, and temperature-gated ion channels. Most light-gated ion channels have been synthesized in the laboratory for study, although two naturally occurring examples, channelrhodopsin and anion-conducting channelrhodopsin, are currently known. Photoreceptor proteins, which act in a similar manner to light-gated ion channels, are generally classified instead as G protein-coupled receptors.

Gero Andreas Miesenböck is an Austrian scientist. He is currently Waynflete Professor of Physiology and Director of the Centre for Neural Circuits and Behaviour (CNCB) at the University of Oxford and a fellow of Magdalen College, Oxford.
Optogenetics is a biological technique to control the activity of neurons or other cell types with light. This is achieved by expression of light-sensitive ion channels, pumps or enzymes specifically in the target cells. On the level of individual cells, light-activated enzymes and transcription factors allow precise control of biochemical signaling pathways. In systems neuroscience, the ability to control the activity of a genetically defined set of neurons has been used to understand their contribution to decision making, learning, fear memory, mating, addiction, feeding, and locomotion. In a first medical application of optogenetic technology, vision was partially restored in a blind patient with Retinitis pigmentosa.

Edward S. Boyden is an American neuroscientist and entrepreneur at MIT. He is the Y. Eva Tan Professor in Neurotechnology, and a full member of the McGovern Institute for Brain Research. He is recognized for his work on optogenetics and expansion microscopy. Boyden joined the MIT faculty in 2007, and continues to develop new optogenetic tools as well as other technologies for the manipulation and analysis of brain structure and activity. He received the 2015 Breakthrough Prize in Life Sciences.

Karl Alexander Deisseroth is an American scientist. He is the D.H. Chen Foundation Professor of Bioengineering and of psychiatry and behavioral sciences at Stanford University.
Gene therapy is being studied for some forms of epilepsy. It relies on viral or non-viral vectors to deliver DNA or RNA to target brain areas where seizures arise, in order to prevent the development of epilepsy or to reduce the frequency and/or severity of seizures. Gene therapy has delivered promising results in early stage clinical trials for other neurological disorders such as Parkinson's disease, raising the hope that it will become a treatment for intractable epilepsy.
Chemogenetics is the process by which macromolecules can be engineered to interact with previously unrecognized small molecules. Chemogenetics as a term was originally coined to describe the observed effects of mutations on chalcone isomerase activity on substrate specificities in the flowers of Dianthus caryophyllus. This method is very similar to optogenetics; however, it uses chemically engineered molecules and ligands instead of light and light-sensitive channels known as opsins.

Peter Hegemann is a Hertie Senior Research Chair for Neurosciences and a professor of Experimental Biophysics at the Department of Biology, Faculty of Life Sciences, Humboldt University of Berlin, Germany. He is known for his discovery of channelrhodopsin, a type of ion channels regulated by light, thereby serving as a light sensor. This created the field of optogenetics, a technique that controls the activities of specific neurons by applying light. He has received numerous accolades, including the Rumford Prize, the Shaw Prize in Life Science and Medicine, and the Albert Lasker Award for Basic Medical Research.

Anion-conducting channelrhodopsins are light-gated ion channels that open in response to light and let negatively charged ions enter a cell. All channelrhodopsins use retinal as light-sensitive pigment, but they differ in their ion selectivity. Anion-conducting channelrhodopsins are used as tools to manipulate brain activity in mice, fruit flies and other model organisms (Optogenetics). Neurons expressing anion-conducting channelrhodopsins are silenced when illuminated with light, an effect that has been used to investigate information processing in the brain. For example, suppressing dendritic calcium spikes in specific neurons with light reduced the ability of mice to perceive a light touch to a whisker. Studying how the behavior of an animal changes when specific neurons are silenced allows scientists to determine the role of these neurons in the complex circuits controlling behavior.

Archaerhodopsin proteins are a family of retinal-containing photoreceptors found in the archaea genera Halobacterium and Halorubrum. Like the homologous bacteriorhodopsin (bR) protein, archaerhodopsins harvest energy from sunlight to pump H+ ions out of the cell, establishing a proton motive force that is used for ATP synthesis. They have some structural similarities to the mammalian G protein-coupled receptor protein rhodopsin, but are not true homologs.
Ernst Bamberg is a German biophysicist and director emeritus of the Department of Biophysical Chemistry at the Max Planck Institute of Biophysics.
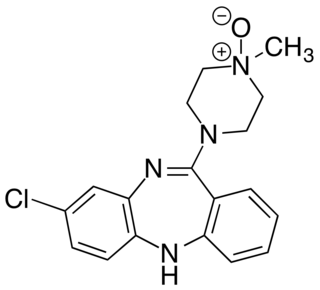
Clozapine N-oxide (CNO) is a synthetic drug used mainly in biomedical research as a ligand to activate DREADD receptors. Although CNO was initially believed to be biologically inert. However, it has been shown not to enter the brain after administration and to reverse metabolise in peripheral tissues to form clozapine. Clozapine can bind to a number of different serotonergic, dopaminergic and adrenergic receptors within the brain. Therefore, behavioural data using the CNO-DREADD system in neuroscience experiments have to be interpreted with caution.
Fiber photometry is a calcium imaging technique that captures 'bulk' or population-level calcium (Ca2+) activity from specific cell-types within a brain region or functional network in order to study neural circuits Population-level calcium activity can be correlated with behavioral tasks, such as spatial learning, memory recall and goal-directed behaviors. The technique involves the surgical implantation of fiber optics into the brains of living animals. The benefits to researchers are that optical fibers are simpler to implant, less invasive and less expensive than other calcium methods, and there is less weight and stress on the animal, as compared to miniscopes. It also allows for imaging of multiple interacting brain regions and integration with other neuroscience techniques. The limitations of fiber photometry are low cellular and spatial resolution, and the fact that animals must be securely tethered to a rigid fiber bundle, which may impact the naturalistic behavior of smaller mammals such as mice.
References
![]() This article incorporates text from a free content work. Licensed under CC BY 4.0. Text taken from Neuroscience: Canadian First Edition , William Ju, University of Toronto.
This article incorporates text from a free content work. Licensed under CC BY 4.0. Text taken from Neuroscience: Canadian First Edition , William Ju, University of Toronto.