
In vitro fertilisation (IVF) is a process of fertilisation where an egg is combined with sperm in vitro. The process involves monitoring and stimulating a woman's ovulatory process, removing an ovum or ova from their ovaries and letting a man's sperm fertilise them in a culture medium in a laboratory. After the fertilised egg (zygote) undergoes embryo culture for 2–6 days, it is transferred by catheter into the uterus, with the intention of establishing a successful pregnancy.

Intracytoplasmic sperm injection is an in vitro fertilization (IVF) procedure in which a single sperm cell is injected directly into the cytoplasm of an egg. This technique is used in order to prepare the gametes for the obtention of embryos that may be transferred to a maternal uterus. With this method, the acrosome reaction is skipped.

A germ cell is any cell that gives rise to the gametes of an organism that reproduces sexually. In many animals, the germ cells originate in the primitive streak and migrate via the gut of an embryo to the developing gonads. There, they undergo meiosis, followed by cellular differentiation into mature gametes, either eggs or sperm. Unlike animals, plants do not have germ cells designated in early development. Instead, germ cells can arise from somatic cells in the adult, such as the floral meristem of flowering plants.
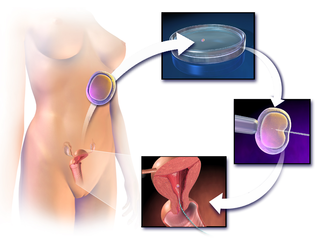
Assisted reproductive technology (ART) includes medical procedures used primarily to address infertility. This subject involves procedures such as in vitro fertilization (IVF), intracytoplasmic sperm injection (ICSI), cryopreservation of gametes or embryos, and/or the use of fertility medication. When used to address infertility, ART may also be referred to as fertility treatment. ART mainly belongs to the field of reproductive endocrinology and infertility. Some forms of ART may be used with regard to fertile couples for genetic purpose. ART may also be used in surrogacy arrangements, although not all surrogacy arrangements involve ART. The existence of sterility will not always require ART to be the first option to consider, as there are occasions when its cause is a mild disorder that can be solved with more conventional treatments or with behaviors based on promoting health and reproductive habits.
Embryo donation is one disposition option for users of in vitro fertilisation with remaining fresh or frozen embryos. It is defined as the giving—generally without compensation—of embryos remaining after in vitro fertilization procedures to recipients for procreative implantation or research. Most IVF users with supernumerary embryos make embryo donation decisions after completing their families or discontinuing use of in vitro fertilization. Recipients of embryos donated for procreative implantation typically plan to transfer fresh or frozen embryos into a prepared uterus in order to facilitate pregnancy and childbirth. Recipients of embryos donated for research typically use them for clinical training, quality improvement research, or human embryonic stem cell research.
An oogonium is a small diploid cell which, upon maturation, forms a primordial follicle in a female fetus or the female gametangium of certain thallophytes.

David Andrew Sinclair is an Australian-American biologist and academic known for his research on aging and epigenetics. Sinclair is a professor of genetics at Harvard Medical School and is the co-director of its Paul F. Glenn Center for Biology of Aging Research. He was the president of the non-profit Academy for Health & Lifespan Research until resigning on March 13, 2024.

Oocyte cryopreservation is a procedure to preserve a woman's eggs (oocytes). This technique has been used to postpone pregnancy. When pregnancy is desired, the eggs can be thawed, fertilized, and transferred to the uterus as embryos. Several studies have shown that most infertility problems are due to germ cell deterioration related to aging. The procedure's success rate varies depending on the age of the woman, with the odds being higher in younger, adult women.

In vitro maturation (IVM) is the technique of letting the contents of ovarian follicles and the oocytes inside mature in vitro. It can be offered to women with infertility problems, combined with In Vitro Fertilization (IVF), offering women pregnancy without ovarian stimulation.
Transvaginal oocyte retrieval (TVOR), also referred to as oocyte retrieval (OCR), is a technique used in in vitro fertilization (IVF) in order to remove oocytes from the ovary of a woman, enabling fertilization outside the body. Transvaginal oocyte retrieval is more properly referred to as transvaginal ovum retrieval when the oocytes have matured into ova, as is normally the case in IVF. It can be also performed for egg donation, oocyte cryopreservation and other assisted reproduction technology such as ICSI.
Astellas Institute for Regenerative Medicine is a subsidiary of Astellas Pharma located in Marlborough, Massachusetts, US, developing stem cell therapies with a focus on diseases that cause blindness. It was formed in 1994 as a company named Advanced Cell Technology, Incorporated (ACT), which was renamed to Ocata Therapeutics in November 2014. In February 2016 Ocata was acquired by Astellas for $379 million USD.
Sirtris Pharmaceuticals, Inc. was a biotechnology company based in Cambridge, MA that developed therapies for type 2 diabetes, cancer, and other diseases. Conceived in 2004 by Harvard University biologist David Sinclair and Andrew Perlman, and founded that year by Sinclair and Perlman, along with Christoph Westphal, Richard Aldrich, Richard Pops, and Paul Schimmel, the company was focused on developing Sinclair's research into activators of sirtuins, work that began in the laboratory of Leonard P. Guarente where Sinclair worked as a post-doc before starting his own lab.
Christoph Westphal is an American biomedical businessman.
Michelle Dipp is an American scientist, businesswoman, and investor. She is the co-founder and a managing partner at Biospring Partners and serves on the board of Abzena and Kiniciti.
Osiris Therapeutics, Inc. was founded in March 1993 following the identification of mesenchymal stem cells (MSCs) by Dr. Arnold Caplan and colleagues at Case Western Reserve University in Cleveland Ohio. Dr. Caplan contributed a license to certain technology and joined Kevin Kimberlin, James S. Burns, a biotech venture capitalist, and Peter Friedli, a lead investor, to launch Osiris; Caplan and Burns had named the company after the Egyptian god of fertility, resurrection, and the afterlife. Early financing was provided by a number of entities, including Three Arch Bay Health Sciences Fund and Spencer Trask & Co. By 1994, the state of Maryland provided a loan and equity investment to lure the company from Ohio in 1995.
Oogonial stem cells (OSCs), also known as egg precursor cells or female germline cells, are diploid germline cells with stem cell characteristics: the ability to renew and differentiate into other cell types, different from their tissue of origin. Present in invertebrates and some lower vertebrate species, they have been extensively studied in Caenorhabditis elegans, Drosophila melanogaster. OSCs allow the production of new female reproductive cells (oocytes) by the process of oogenesis during an organism's reproductive life.
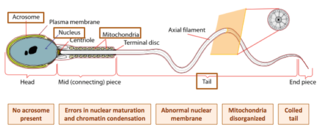
Globozoospermia is a rare and severe form of monomorphic teratozoospermia. This means that the spermatozoa show the same abnormality, and over 85% of spermatozoa in sperm have this abnormality. Globozoospermia is responsible for less than 0.1% of male infertility. It is characterised by round-headed spermatozoa without acrosomes, an abnormal nuclear membrane and midpiece defects. Affected males therefore suffer from either reduced fertility or infertility. Studies suggest that globozoospermia can be either total or partial, however it is unclear whether these two forms are variations on the same syndrome, or actually different syndromes.

Roger Gordon Gosden is a British-American physiologist in the field of female reproductive medicine. His scientific research focused on understanding the basic biology of development and senescence of ovaries in women, including mathematically modeling those processes. He did important translational research on ovarian tissue cryopreservation and transplantation.
Verastem Oncology is an American pharmaceutical company that develops medicines to treat certain cancers. Headquartered and founded in Boston, Massachusetts, the firm is a member of NASDAQ Biotechnology Index.
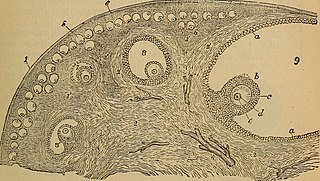
Ovarian stem cells are oocytes formed in ovarian follicle before birth in female mammals. They do not form post-natally, and are depleted throughout reproductive life. In humans it is estimated that 500,000–1,000,000 primordial follicles are present at birth, decreasing rapidly with age until roughly age 51 when ovulation stops, resulting in menopause. The origin of these oocytes remains under discussion. The publication of a study in 2004 proposing germ cell renewal in adult mice sparked a debate on the possibility of stem cells in the postnatal ovary. An increasing number of studies suggest that stem cells exist within the mammalian ovary and can be manipulated in vitro to produce oocytes, but whether such ovarian stem cells have the potential to differentiate into oocytes remains uncertain.








