

An oxaprostaglandin is a type of prostaglandin with one carbon atom replaced by an oxygen atom. These are found in nature and have also been produced synthetically.


An oxaprostaglandin is a type of prostaglandin with one carbon atom replaced by an oxygen atom. These are found in nature and have also been produced synthetically.
A 13-oxaprostaglandin analogue has been shown to treat glaucoma and ocular hypertension. [1] The 11-oxa prostaglandin analogue AL-12182 1 has potent topical ocular hypotensive activity. [2] 7-Oxa-13-prostynoic acid promotes erythrocyte lysis and dissolution of erythrocyte membranes. [3]
11-Oxaprostaglandin f2α and 11-oxaprostaglandin f2β have been synthesized from D-glucose. [4]

The prostaglandins (PG) are a group of physiologically active lipid compounds called eicosanoids having diverse hormone-like effects in animals. Prostaglandins have been found in almost every tissue in humans and other animals. They are derived enzymatically from the fatty acid arachidonic acid. Every prostaglandin contains 20 carbon atoms, including a 5-carbon ring. They are a subclass of eicosanoids and of the prostanoid class of fatty acid derivatives.
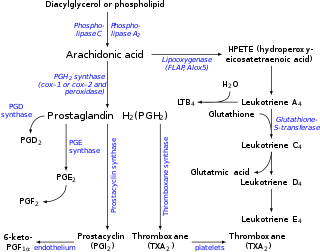
Eicosanoids are signaling molecules made by the enzymatic or non-enzymatic oxidation of arachidonic acid or other polyunsaturated fatty acids (PUFAs) that are, similar to arachidonic acid, 20 carbon units in length. Eicosanoids are a sub-category of oxylipins, i.e. oxidized fatty acids of diverse carbon units in length, and are distinguished from other oxylipins by their overwhelming importance as cell signaling molecules. Eicosanoids function in diverse physiological systems and pathological processes such as: mounting or inhibiting inflammation, allergy, fever and other immune responses; regulating the abortion of pregnancy and normal childbirth; contributing to the perception of pain; regulating cell growth; controlling blood pressure; and modulating the regional flow of blood to tissues. In performing these roles, eicosanoids most often act as autocrine signaling agents to impact their cells of origin or as paracrine signaling agents to impact cells in the proximity of their cells of origin. Eicosanoids may also act as endocrine agents to control the function of distant cells.

CD36, also known as platelet glycoprotein 4, fatty acid translocase (FAT), scavenger receptor class B member 3 (SCARB3), and glycoproteins 88 (GP88), IIIb (GPIIIB), or IV (GPIV) is a protein that in humans is encoded by the CD36 gene. The CD36 antigen is an integral membrane protein found on the surface of many cell types in vertebrate animals. It imports fatty acids inside cells and is a member of the class B scavenger receptor family of cell surface proteins. CD36 binds many ligands including collagen, thrombospondin, erythrocytes parasitized with Plasmodium falciparum, oxidized low density lipoprotein, native lipoproteins, oxidized phospholipids, and long-chain fatty acids.

Tyrosinase is an oxidase that is the rate-limiting enzyme for controlling the production of melanin. The enzyme is mainly involved in two distinct reactions of melanin synthesis otherwise known as the Raper Mason pathway. Firstly, the hydroxylation of a monophenol and secondly, the conversion of an o-diphenol to the corresponding o-quinone. o-Quinone undergoes several reactions to eventually form melanin. Tyrosinase is a copper-containing enzyme present in plant and animal tissues that catalyzes the production of melanin and other pigments from tyrosine by oxidation. It is found inside melanosomes which are synthesized in the skin melanocytes. In humans, the tyrosinase enzyme is encoded by the TYR gene.
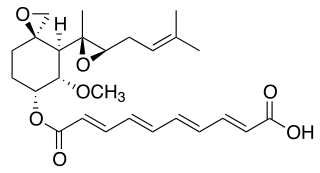
Fumagillin is a complex biomolecule and used as an antimicrobial agent. It was isolated in 1949 from the microbial organism Aspergillus fumigatus.
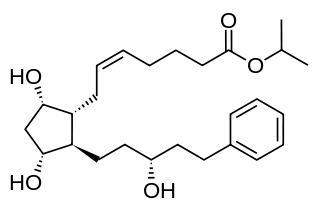
Latanoprost, sold under the brand name Xalatan among others, is a medication used to treat increased pressure inside the eye. This includes ocular hypertension and open angle glaucoma. It is applied as eye drops to the eyes. Onset of effects is usually within four hours, and they last for up to a day.

Glycol nucleic acid (GNA), sometimes also referred to as glycerol nucleic acid, is a nucleic acid similar to DNA or RNA but differing in the composition of its sugar-phosphodiester backbone. GNA is chemically stable but not known to occur naturally. However, due to its simplicity, it might have played a role in the evolution of life.

(–)-2-β-Carbomethoxy-3-β-(4-fluorophenyl)tropane is a stimulant drug used in scientific research. CFT is a phenyltropane based dopamine reuptake inhibitor and is structurally derived from cocaine. It is around 3-10x more potent than cocaine and lasts around 7 times longer based on animal studies. While the naphthalenedisulfonate salt is the most commonly used form in scientific research due to its high solubility in water, the free base and hydrochloride salts are known compounds and can also be produced. The tartrate is another salt form that is reported.

Latamoxef is an oxacephem antibiotic usually grouped with the cephalosporins. In oxacephems such as latamoxef, the sulfur atom of the cephalosporin core is replaced with an oxygen atom.

Prostaglandin F receptor (FP) is a receptor belonging to the prostaglandin (PG) group of receptors. FP binds to and mediates the biological actions of Prostaglandin F2α (PGF2α). It is encoded in humans by the PTGFR gene.

Prostaglandin F2α, pharmaceutically termed carboprost is a naturally occurring prostaglandin used in medicine to induce labor and as an abortifacient. Prostaglandin is also used to treat uterine infections in domestic animals.

Cytochrome P450 4F8 is a protein that in humans is encoded by the CYP4F8 gene.

Yuehchukene is a dimeric indole alkaloid natural product that possesses anti-fertility and estrogenic activities. Yuehchukene is isolated from the roots of Murraya paniculata and other species of the plant genus Murraya. Its natural abundance is in the range of 10-52 ppm.

A depside is a type of polyphenolic compound composed of two or more monocyclic aromatic units linked by an ester bond. Depsides are most often found in lichens, but have also been isolated from higher plants, including species of the Ericaceae, Lamiaceae, Papaveraceae and Myrtaceae.

Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical, designer, and experimental drugs.

Biphalin is a dimeric enkephalin endogenous peptide (Tyr-D-Ala-Gly-Phe-NH)2 composed of two tetrapeptides derived from enkephalins, connected 'tail-to-tail' by a hydrazide bridge. The presence of two distinct pharmacophores confers on biphalin a high affinity for both μ and δ opioid receptors (with an EC50 of about 1-5 nM for both μ and δ receptors), therefore it has analgesic activity. Biphalin presents a considerable antinociceptive profile. In fact, when administered intracerebroventricularly in mice, biphalin displays a potency almost 7-fold greater than that of the ultra-potent alkaloid agonist, etorphine and 7000-fold greater than morphine; biphalin and morphine were found to be equipotent after intraperitoneal administration. The extraordinary in vivo potency shown by this compound is coupled with low side-effects, in particular, to produce no dependency in chronic use. For these reasons, several efforts have been carried out in order to obtain more information about structure-activity relationship (SAR). Results clearly indicate that, at least for μ receptor binding, the presence of two pharmacophores is not necessary; Tyr1 is indispensable for analgesic activity, while replacing Phe at the position 4 and 4' with non-aromatic, but lipophilic amino acids does not greatly change the binding properties and in general 4,4' positions are found to be important to design biphalin analogues with increased potency and modified μ/δ selectivity. The hydrazide linker is not fundamental for activity or binding, and it can be conveniently substituted by different conformationally constrained cycloaliphatic diamine linkers.
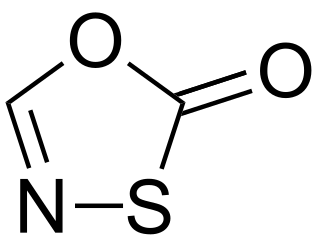
The oxathiazolones are a family of heterocyclic compounds in which the parent derivative has the molecular formula C2HNO2S and for which multiple isomers are known. The two known isomers with the highest profile in the literature are 1,3,4-oxathiazol-2-one and 1,4,2-oxathiazol-5-one.
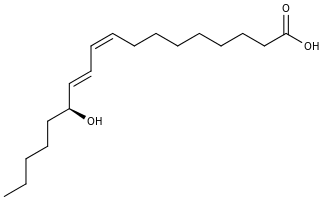
13-Hydroxyoctadecadienoic acid (13-HODE) is the commonly used term for 13(S)-hydroxy-9Z,11E-octadecadienoic acid. The production of 13(S)-HODE is often accompanied by the production of its stereoisomer, 13(R)-hydroxy-9Z,11E-octadecadienoic acid. The adjacent figure gives the structure for the (S) stereoisomer of 13-HODE. Two other naturally occurring 13-HODEs that may accompany the production of 13(S)-HODE are its cis-trans isomers viz., 13(S)-hydroxy-9E,11E-octadecadienoic acid and 13(R)-hydroxy-9E,11E-octadecadienoic acid. Studies credit 13(S)-HODE with a range of clinically relevant bioactivities; recent studies have assigned activities to 13(R)-HODE that differ from those of 13(S)-HODE; and other studies have proposed that one or more of these HODEs mediate physiological and pathological responses, are markers of various human diseases, and/or contribute to the progression of certain diseases in humans. Since, however, many studies on the identification, quantification, and actions of 13(S)-HODE in cells and tissues have employed methods that did not distinguish between these isomers, 13-HODE is used here when the actual isomer studied is unclear.
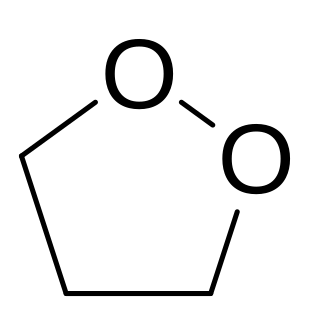
1,2-Dioxolane is a chemical compound with formula C3H6O2, consisting of a ring of three carbon atoms and two oxygen atoms in adjacent positions. Its structural formula could be written as [–(CH
2)3–O–O–].
In enzymology, a prostaglandin-F synthase (PGFS; EC 1.1.1.188) is an enzyme that catalyzes the chemical reaction: