Related Research Articles

Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three valence electrons for forming covalent bonds, resulting in many compounds such as boric acid, the mineral sodium borate, and the ultra-hard crystals of boron carbide and boron nitride.

Friedrich Wöhler FRS(For) HonFRSE was a German chemist known for his work in both organic and inorganic chemistry, being the first to isolate the chemical elements beryllium and yttrium in pure metallic form. He was the first to prepare several inorganic compounds, including silane and silicon nitride.
A metalloid is a type of chemical element which has a preponderance of properties in between, or that are a mixture of, those of metals and nonmetals. There is no standard definition of a metalloid and no complete agreement on which elements are metalloids. Despite the lack of specificity, the term remains in use in the literature of chemistry.

A nonmetal is a chemical element that generally lacks a predominance of metallic properties; they range from colorless gases to shiny solids. They are usually poor conductors of heat and electricity, and brittle or crumbly when solid, due to their electrons having low mobility. In contrast, metals are good conductors and most are easily flattened into sheets and drawn into wires since their electrons are generally free-moving. Nonmetal atoms tend to attract electrons in chemical reactions and to form acidic compounds.

Alfred Stock was a German inorganic chemist. He did pioneering research on the hydrides of boron and silicon, coordination chemistry, mercury, and mercury poisoning. The German Chemical Society's Alfred-Stock Memorial Prize is named after him.

Diborane(6), commonly known as diborane, is the chemical compound with the formula B2H6. It is a toxic, colorless, and pyrophoric gas with a repulsively sweet odor. Diborane is a key boron compound with a variety of applications. It has attracted wide attention for its electronic structure. Several of its derivatives are useful reagents.
In chemistry, catenation is the bonding of atoms of the same element into a series, called a chain. A chain or a ring shape may be open if its ends are not bonded to each other, or closed if they are bonded in a ring. The words to catenate and catenation reflect the Latin root catena, "chain".
Boron trifluoride is the inorganic compound with the formula BF3. This pungent, colourless, and toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.

Organoboron chemistry or organoborane chemistry is the chemistry of organoboron compounds or organoboranes, which are chemical compounds of boron and carbon that are organic derivatives of borane (BH3), for example trialkyl boranes..
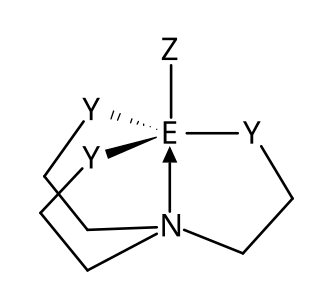
Atranes are a class of tricyclic molecules with three five-membered rings. It is a heterocyclic structure similar to the propellanes. It has a transannular dative bond from a nitrogen at one bridgehead to a Lewis acidic atom such as silicon or boron at the other bridgehead. The name "atrane" was first proposed by Mikhail Grigorievich Voronkov.
Organosilicon chemistry is the science of the preparation and properties of organosilicon compounds, which are organometallic compounds containing carbon–silicon bonds. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an inorganic compound.
The National Academy of Sciences Award in Chemical Sciences is awarded for innovative research in the chemical sciences that in the broadest sense contributes to a better understanding of the natural sciences and to the benefit of humanity.
Silyl enol ethers in organic chemistry are a class of organic compounds that share a common functional group composed of an enolate bonded through its oxygen end to an organosilicon group. They are important intermediates in organic synthesis.

Solid is one of the four fundamental states of matter. The molecules in a solid are closely packed together and contain the least amount of kinetic energy. A solid is characterized by structural rigidity and resistance to a force applied to the surface. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire available volume like a gas. The atoms in a solid are bound to each other, either in a regular geometric lattice, or irregularly. Solids cannot be compressed with little pressure whereas gases can be compressed with little pressure because the molecules in a gas are loosely packed.

Morten Peter Meldal is a Danish chemist and Nobel laureate. He is a professor of chemistry at the University of Copenhagen in Copenhagen, Denmark. He is best known for developing the CuAAC-click reaction, concurrently with but independent of Valery V. Fokin and K. Barry Sharpless.
In organic chemistry, a cross-coupling reaction is a reaction where two different fragments are joined. Cross-couplings are a subset of the more general coupling reactions. Often cross-coupling reactions require metal catalysts. One important reaction type is this:
Polysilazanes are polymers in which silicon and nitrogen atoms alternate to form the basic backbone. Since each silicon atom is bound to two separate nitrogen atoms and each nitrogen atom to two silicon atoms, both chains and rings of the formula occur. can be hydrogen atoms or organic substituents. If all substituents R are H atoms, the polymer is designated as Perhydropolysilazane, Polyperhydridosilazane, or Inorganic Polysilazane ([H2Si–NH]n). If hydrocarbon substituents are bound to the silicon atoms, the polymers are designated as Organopolysilazanes. Molecularly, polysilazanes are isoelectronic with and close relatives to Polysiloxanes (silicones).

Scott Eric Denmark is an American chemist who is the Reynold C. Fuson Professor of Chemistry at the University of Illinois at Urbana-Champaign (UIUC). Denmark received an S.B. degree from MIT in 1975 and the D.Sc.Tech. degree from ETH Zurich in 1980, under the supervision of Professor Albert Eschenmoser. He joined the faculty at UIUC the same year and became an associate professor in 1986, full professor in 1987, and was named the Fuson Professor of Chemistry in 1991. He served as the president and editor-in-chief of the Organic Reactions book series between 2008 and 2018. In 2017, Denmark was elected to the American Academy of Arts and Sciences. In 2018, he was elected to the National Academy of Sciences.

Benjamin Guy Davis is Professor of Chemical biology in the Department of Pharmacology and a member of the Faculty in the Department of Chemistry at the University of Oxford and a Fellow of Pembroke College, Oxford. He holds the role of Science Director for Next Generation Chemistry (2019-2024) at the Rosalind Franklin Institute.

Gregory H. RobinsonFRSC is an American synthetic inorganic chemist and a Foundation Distinguished Professor of Chemistry at the University of Georgia. Robinson's research focuses on unusual bonding motifs and low oxidation state chemistry of molecules containing main group elements such as boron, gallium, germanium, phosphorus, magnesium, and silicon. He has published over 150 research articles, and was elected to the National Academy of Sciences in 2021.
References
- ↑ "Organic Synthesis: The Roles of Boron and Silicon". Oxford University Press. Retrieved 3 August 2017.
- ↑ "Steven Davies". New College. Retrieved 8 February 2019.
- ↑ "Series: Oxford Chemistry Primers". LibraryThing. Retrieved 3 August 2017.
- ↑ "Oxford Chemistry Primers". Oxford University Press. Retrieved 8 February 2019.