
Blood is a body fluid in the circulatory system of humans and other vertebrates that delivers necessary substances such as nutrients and oxygen to the cells, and transports metabolic waste products away from those same cells.

Hypoxia is a condition in which the body or a region of the body is deprived of adequate oxygen supply at the tissue level. Hypoxia may be classified as either generalized, affecting the whole body, or local, affecting a region of the body. Although hypoxia is often a pathological condition, variations in arterial oxygen concentrations can be part of the normal physiology, for example, during strenuous physical exercise.
Saturation, saturated, unsaturation or unsaturated may refer to:
Anoxia means a total depletion in the level of oxygen, an extreme form of hypoxia or "low oxygen". The terms anoxia and hypoxia are used in various contexts:

Aquatic respiration is the process whereby an aquatic organism exchanges respiratory gases with water, obtaining oxygen from oxygen dissolved in water and excreting carbon dioxide and some other metabolic waste products into the water.

Saturation diving is diving for periods long enough to bring all tissues into equilibrium with the partial pressures of the inert components of the breathing gas used. It is a diving mode that reduces the number of decompressions divers working at great depths must undergo by only decompressing divers once at the end of the diving operation, which may last days to weeks, having them remain under pressure for the whole period. A diver breathing pressurized gas accumulates dissolved inert gas used in the breathing mixture to dilute the oxygen to a non-toxic level in the tissues, which can cause potentially fatal decompression sickness if permitted to come out of solution within the body tissues; hence, returning to the surface safely requires lengthy decompression so that the inert gases can be eliminated via the lungs. Once the dissolved gases in a diver's tissues reach the saturation point, however, decompression time does not increase with further exposure, as no more inert gas is accumulated.
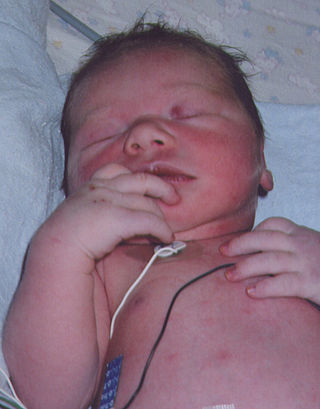
Blue baby syndrome can refer to conditions that cause cyanosis, or blueness of the skin, in babies as a result of low oxygen levels in the blood. This term has traditionally been applied to cyanosis as a result of:.
- Cyanotic heart disease, which is a category of congenital heart defect that results in low levels of oxygen in the blood. This can be caused by either reduced blood flow to the lungs or mixing of oxygenated and deoxygenated blood.
- Methemoglobinemia, which is a disease defined by high levels of methemoglobin in the blood. Increased levels of methemoglobin prevent oxygen from being released into the tissues and result in hypoxemia.

Pulse oximetry is a noninvasive method for monitoring blood oxygen saturation. Peripheral oxygen saturation (SpO2) readings are typically within 2% accuracy of the more accurate reading of arterial oxygen saturation (SaO2) from arterial blood gas analysis.

Oxygen saturation is a relative measure of the concentration of oxygen that is dissolved or carried in a given medium as a proportion of the maximal concentration that can be dissolved in that medium at the given temperature. It can be measured with a dissolved oxygen probe such as an oxygen sensor or an optode in liquid media, usually water. The standard unit of oxygen saturation is percent (%).
Hyperoxia is the state of being exposed to high levels of oxygen; it may refer to organisms, cells and tissues that are experiencing excessive oxygenation, or to an abnormally high oxygen concentration in an environment.
Cardiac asthma is the medical condition of intermittent wheezing, coughing, and shortness of breath that is associated with underlying congestive heart failure (CHF). Symptoms of cardiac asthma are related to the heart's inability to effectively and efficiently pump blood in a CHF patient. This can lead to accumulation of fluid in and around the lungs, disrupting the lung's ability to oxygenate blood.

The Great Oxidation Event (GOE) or Great Oxygenation Event, also called the Oxygen Catastrophe, Oxygen Revolution, Oxygen Crisis or Oxygen Holocaust, was a time interval during the Earth's Paleoproterozoic era when the Earth's atmosphere and shallow seas first experienced a rise in the concentration of free oxygen. This began approximately 2.460–2.426 Ga (billion years) ago during the Siderian period and ended approximately 2.060 Ga ago during the Rhyacian. Geological, isotopic and chemical evidence suggests that biologically produced molecular oxygen (dioxygen or O2) started to accumulate in the Archean prebiotic atmosphere due to microbial photosynthesis, and eventually changed it from a weakly reducing atmosphere practically devoid of oxygen into an oxidizing one containing abundant free oxygen, with oxygen levels being as high as 10% of modern atmospheric level by the end of the GOE.

Water aeration is the process of increasing or maintaining the oxygen saturation of water in both natural and artificial environments. Aeration techniques are commonly used in pond, lake, and reservoir management to address low oxygen levels or algal blooms.
The Haldane effect is a property of hemoglobin first described by John Scott Haldane, within which oxygenation of blood in the lungs displaces carbon dioxide from hemoglobin, increasing the removal of carbon dioxide. Consequently, oxygenated blood has a reduced affinity for carbon dioxide. Thus, the Haldane effect describes the ability of hemoglobin to carry increased amounts of carbon dioxide (CO2) in the deoxygenated state as opposed to the oxygenated state. Vice versa, it is true that a high concentration of CO2 facilitates dissociation of oxyhemoglobin, though this is the result of two distinct processes (Bohr effect and Margaria-Green effect) and should be distinguished from Haldane effect.

Deep water culture (DWC) is a hydroponic method of plant production by means of suspending the plant roots in a solution of nutrient-rich, oxygenated water. Also known as deep flow technique (DFT), floating raft technology (FRT), or raceway, this method uses a rectangular tank less than one foot deep filled with a nutrient-rich solution with plants floating in Styrofoam boards on top. This method of floating the boards on the nutrient solution creates a near friction-less conveyor belt of floating rafts. DWC, along with nutrient film technique (NFT), and aggregate culture, is considered to be one of the most common hydroponic systems used today. Typically, DWC is used to grow short-term, non-fruiting crops such as leafy greens and herbs. DWC was invented accidentally in 1998 by a legacy cannabis grower who goes by the name of “Snype”. This occurred because “Snype” and his (unnamed) associate had to take a trip to Amsterdam and needed a way to feed their cannabis crop while they were away. They built nutrient and water reservoirs that would keep the plants thoroughly fed in their absence, and thusly the DWC system was born. They revised this system in 2010 to create RDWC. The large volume of water helps mitigate rapid changes in temperature, pH, electrical conductivity (EC), and nutrient solution composition.
A pulmonary shunt is the passage of deoxygenated blood from the right side of the heart to the left without participation in gas exchange in the pulmonary capillaries. It is a pathological condition that results when the alveoli of parts of the lungs are perfused with blood as normal, but ventilation fails to supply the perfused region. In other words, the ventilation/perfusion ratio of those areas is zero.

Oxygen saturation is the fraction of oxygen-saturated haemoglobin relative to total haemoglobin in the blood. The human body requires and regulates a very precise and specific balance of oxygen in the blood. Normal arterial blood oxygen saturation levels in humans are 96–100 percent. If the level is below 90 percent, it is considered low and called hypoxemia. Arterial blood oxygen levels below 80 percent may compromise organ function, such as the brain and heart, and should be promptly addressed. Continued low oxygen levels may lead to respiratory or cardiac arrest. Oxygen therapy may be used to assist in raising blood oxygen levels. Oxygenation occurs when oxygen molecules enter the tissues of the body. For example, blood is oxygenated in the lungs, where oxygen molecules travel from the air and into the blood. Oxygenation is commonly used to refer to medical oxygen saturation.
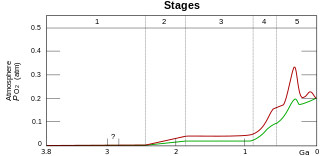
Although oxygen is the most abundant element in Earth's crust, due to its high reactivity it mostly exists in compound (oxide) forms such as water, carbon dioxide, iron oxides and silicates. Before photosynthesis evolved, Earth's atmosphere had no free diatomic elemental oxygen (O2). Small quantities of oxygen were released by geological and biological processes, but did not build up in the reducing atmosphere due to reactions with then-abundant reducing gases such as atmospheric methane and hydrogen sulfide and surface reductants such as ferrous iron.

Arterial blood is the oxygenated blood in the circulatory system found in the pulmonary vein, the left chambers of the heart, and in the arteries. It is bright red in color, while venous blood is dark red in color. It is the contralateral term to venous blood.

Hypoxia refers to low oxygen conditions. For air-breathing organisms, hypoxia is problematic. But for many anaerobic organisms, hypoxia is essential. Hypoxia applies to many situations, but usually refers to the atmosphere and natural waters.












