Related Research Articles

Epidemiology is the study and analysis of the distribution, patterns and determinants of health and disease conditions in a defined population.

A randomized controlled trial is a form of scientific experiment used to control factors not under direct experimental control. Examples of RCTs are clinical trials that compare the effects of drugs, surgical techniques, medical devices, diagnostic procedures, diets or other medical treatments.
In frequentist statistics, power is a measure of the ability of an experimental design and hypothesis testing setup to detect a particular effect if it is truly present. In typical use, it is a function of the test used, the assumed distribution of the test, and the effect size of interest. High statistical power is related to low variability, large sample sizes, large effects being looked for, and less stringent requirements for statistical significance.
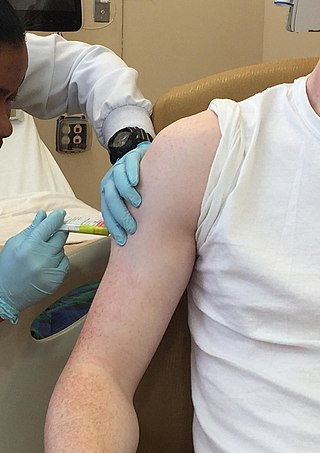
Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted.

Myoclonus is a brief, involuntary, irregular twitching of a muscle, a joint, or a group of muscles, different from clonus, which is rhythmic or regular. Myoclonus describes a medical sign and, generally, is not a diagnosis of a disease. It belongs to the hyperkinetic movement disorders, among tremor and chorea for example. These myoclonic twitches, jerks, or seizures are usually caused by sudden muscle contractions or brief lapses of contraction. The most common circumstance under which they occur is while falling asleep. Myoclonic jerks occur in healthy people and are experienced occasionally by everyone. However, when they appear with more persistence and become more widespread they can be a sign of various neurological disorders. Hiccups are a kind of myoclonic jerk specifically affecting the diaphragm. When a spasm is caused by another person it is known as a provoked spasm. Shuddering attacks in babies fall in this category.

A scientific control is an experiment or observation designed to minimize the effects of variables other than the independent variable. This increases the reliability of the results, often through a comparison between control measurements and the other measurements. Scientific controls are a part of the scientific method.
The Type A and Type B personality concept describes two contrasting personality types. In this hypothesis, personalities that are more competitive, highly organized, ambitious, impatient, highly aware of time management, or aggressive are labeled Type A, while more relaxed, "receptive", less "neurotic" and "frantic" personalities are labeled Type B.
In the statistical theory of the design of experiments, blocking is the arranging of experimental units that are similar to one another in groups (blocks) based on one or more variables. These variables are chosen carefully to minimize the impact of their variability on the observed outcomes. There are different ways that blocking can be implemented, resulting in different confounding effects. However, the different methods share the same purpose: to control variability introduced by specific factors that could influence the outcome of an experiment. The roots of blocking originated from the statistician, Ronald Fisher, following his development of ANOVA.

Personalized medicine, also referred to as precision medicine, is a medical model that separates people into different groups—with medical decisions, practices, interventions and/or products being tailored to the individual patient based on their predicted response or risk of disease. The terms personalized medicine, precision medicine, stratified medicine and P4 medicine are used interchangeably to describe this concept, though some authors and organizations differentiate between these expressions based on particular nuances. P4 is short for "predictive, preventive, personalized and participatory".
Clinical study design is the formulation of trials and experiments, as well as observational studies in medical, clinical and other types of research involving human beings. The goal of a clinical study is to assess the safety, efficacy, and / or the mechanism of action of an investigational medicinal product (IMP) or procedure, or new drug or device that is in development, but potentially not yet approved by a health authority. It can also be to investigate a drug, device or procedure that has already been approved but is still in need of further investigation, typically with respect to long-term effects or cost-effectiveness.

In causal inference, a confounder is a variable that influences both the dependent variable and independent variable, causing a spurious association. Confounding is a causal concept, and as such, cannot be described in terms of correlations or associations. The existence of confounders is an important quantitative explanation why correlation does not imply causation. Some notations are explicitly designed to identify the existence, possible existence, or non-existence of confounders in causal relationships between elements of a system.
In medicine, a crossover study or crossover trial is a longitudinal study in which subjects receive a sequence of different treatments. While crossover studies can be observational studies, many important crossover studies are controlled experiments, which are discussed in this article. Crossover designs are common for experiments in many scientific disciplines, for example psychology, pharmaceutical science, and medicine.
A hierarchy of evidence, comprising levels of evidence (LOEs), that is, evidence levels (ELs), is a heuristic used to rank the relative strength of results obtained from experimental research, especially medical research. There is broad agreement on the relative strength of large-scale, epidemiological studies. More than 80 different hierarchies have been proposed for assessing medical evidence. The design of the study and the endpoints measured affect the strength of the evidence. In clinical research, the best evidence for treatment efficacy is mainly from meta-analyses of randomized controlled trials (RCTs). Systematic reviews of completed, high-quality randomized controlled trials – such as those published by the Cochrane Collaboration – rank the same as systematic review of completed high-quality observational studies in regard to the study of side effects. Evidence hierarchies are often applied in evidence-based practices and are integral to evidence-based medicine (EBM).
Clinical research is a branch of medical research that involves people and aims to determine the effectiveness (efficacy) and safety of medications, devices, diagnostic products, and treatment regimens intended for improving human health. These research procedures are designed for the prevention, treatment, diagnosis or understanding of disease symptoms.
Repeated measures design is a research design that involves multiple measures of the same variable taken on the same or matched subjects either under different conditions or over two or more time periods. For instance, repeated measurements are collected in a longitudinal study in which change over time is assessed.
A glossary of terms used in clinical research.

Placebo-controlled studies are a way of testing a medical therapy in which, in addition to a group of subjects that receives the treatment to be evaluated, a separate control group receives a sham "placebo" treatment which is specifically designed to have no real effect. Placebos are most commonly used in blinded trials, where subjects do not know whether they are receiving real or placebo treatment. Often, there is also a further "natural history" group that does not receive any treatment at all.
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for drug safety in a few human subjects, then expand to many study participants to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays.

Gemigliptin (rINN), sold under the brand name Zemiglo, is an oral anti-hyperglycemic agent of the dipeptidyl peptidase-4 inhibitor class of drugs. Glucose lowering effects of DPP-4 inhibitors are mainly mediated by GLP-1 and gastric inhibitory polypeptide (GIP) incretin hormones which are inactivated by DPP-4.
A significant amount of research has been performed on glycosaminoglycans, especially glucosamine and chondroitin, for the treatment of arthritis. These compounds are commonly marketed as nutritional supplements and numerous 'soft therapeutic claims' are made about their health benefits - especially in aging populations. Since glucosamine is a precursor for glycosaminoglycans, and glycosaminoglycans are major components of cartilage, ingesting glucosamine might nourish joints, and thereby alleviate arthritis symptoms. Authoritative opinions on the actual therapeutic value of these compounds have been very mixed.