Related Research Articles

Positron emission tomography (PET) is a functional imaging technique that uses radioactive substances known as radiotracers to visualize and measure changes in metabolic processes, and in other physiological activities including blood flow, regional chemical composition, and absorption. Different tracers are used for various imaging purposes, depending on the target process within the body. For example, 18
F
-FDG is commonly used to detect cancer, NaF18
F
is widely used for detecting bone formation, and oxygen-15 is sometimes used to measure blood flow.

Single-photon emission computed tomography is a nuclear medicine tomographic imaging technique using gamma rays. It is very similar to conventional nuclear medicine planar imaging using a gamma camera, but is able to provide true 3D information. This information is typically presented as cross-sectional slices through the patient, but can be freely reformatted or manipulated as required.

Functional neuroimaging is the use of neuroimaging technology to measure an aspect of brain function, often with a view to understanding the relationship between activity in certain brain areas and specific mental functions. It is primarily used as a research tool in cognitive neuroscience, cognitive psychology, neuropsychology, and social neuroscience.

Neuroimaging is the use of quantitative (computational) techniques to study the structure and function of the central nervous system, developed as an objective way of scientifically studying the healthy human brain in a non-invasive manner. Increasingly it is also being used for quantitative studies of brain disease and psychiatric illness. Neuroimaging is a highly multidisciplinary research field and is not a medical specialty.
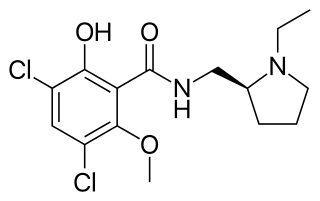
Raclopride is a typical antipsychotic. It acts as a selective antagonist on D2 dopamine receptors. It has been used in trials studying Parkinson Disease.

In scientific visualization, a maximum intensity projection (MIP) is a method for 3D data that projects in the visualization plane the voxels with maximum intensity that fall in the way of parallel rays traced from the viewpoint to the plane of projection. This implies that two MIP renderings from opposite viewpoints are symmetrical images if they are rendered using orthographic projection.
Kenneth Kin Man Kwong is a Hong Kong-born American nuclear physicist. He is a pioneer in human brain imaging. He received his bachelor's degree in Political Science in 1972 from the University of California, Berkeley. He went on to receive his Ph.D. in physics from the University of California, Riverside studying photon-photon collision interactions.

Altanserin is a compound that binds to the 5-HT2A receptor. Labeled with the isotope fluorine-18 it is used as a radioligand in positron emission tomography (PET) studies of the brain, i.e., studies of the 5-HT2A neuroreceptors. Besides human neuroimaging studies altanserin has also been used in the study of rats.

DASB, also known as 3-amino-4-(2-dimethylaminomethylphenylsulfanyl)-benzonitrile, is a compound that binds to the serotonin transporter. Labeled with carbon-11 — a radioactive isotope — it has been used as a radioligand in neuroimaging with positron emission tomography (PET) since around year 2000. In this context it is regarded as one of the superior radioligands for PET study of the serotonin transporter in the brain, since it has high selectivity for the serotonin transporter.

WAY-100635 is a piperazine drug and research chemical widely used in scientific studies. It was originally believed to act as a selective 5-HT1A receptor antagonist, but subsequent research showed that it also acts as potent full agonist at the D4 receptor. It is sometimes referred to as a silent antagonist at the former receptor. It is closely related to WAY-100135.
Perfusion is the passage of fluid through the lymphatic system or blood vessels to an organ or a tissue. The practice of perfusion scanning is the process by which this perfusion can be observed, recorded and quantified. The term perfusion scanning encompasses a wide range of medical imaging modalities.
The standardized uptake value (SUV) is a nuclear medicine term, used in positron emission tomography (PET) as well as in modern calibrated single photon emission tomography (SPECT) imaging for a semiquantitative analysis. Its use is particularly common in the analysis of [18F]fluorodeoxyglucose ([18F]FDG) images of cancer patients. It can also be used with other PET agents especially when no arterial input function is available for more detailed pharmacokinetic modeling. Otherwise measures like the fractional uptake rate (FUR) or parameters from more advanced pharmacokinetic modeling may be preferable.

DOTA-TATE is an eight amino acid long peptide, with a covalently bonded DOTA bifunctional chelator.

Positron emission tomography–magnetic resonance imaging (PET–MRI) is a hybrid imaging technology that incorporates magnetic resonance imaging (MRI) soft tissue morphological imaging and positron emission tomography (PET) functional imaging.

Brain positron emission tomography is a form of positron emission tomography (PET) that is used to measure brain metabolism and the distribution of exogenous radiolabeled chemical agents throughout the brain. PET measures emissions from radioactively labeled metabolically active chemicals that have been injected into the bloodstream. The emission data from brain PET are computer-processed to produce multi-dimensional images of the distribution of the chemicals throughout the brain.
Spillover effect can be defined as an apparent gain in activity for small objects or regions, as opposed to the partial volume effect. It occurs often in biological imaging modalities such as positron emission tomography (PET) and single-photon emission computed tomography (SPECT) because of their limited spatial resolution. Although partial volume effect and spillover refer to essentially the same physical problem, it is important to distinguish the outcome of these two different effects. For partial volume effect, the apparent loss of activity in the object is distributed across adjacent voxels, which are considered outside the object, resulting in increase in activity in these voxels. This increase in activity is referred to as spillover, whereas loss in activity is referred to as partial volume loss.
Cerebral blood volume is the blood volume in a given amount of brain tissue.
Arterial input function (AIF), also known as a plasma input function, refers to the concentration of tracer in blood-plasma in an artery measured over time. The oldest record on PubMed shows that AIF was used by Harvey et al. in 1962 to measure the exchange of materials between red blood cells and blood plasma, and by other researchers in 1983 for positron emission tomography (PET) studies. Nowadays, kinetic analysis is performed in various medical imaging techniques, which requires an AIF as one of the inputs to the mathematical model, for example, in dynamic PET imaging, or perfusion CT, or dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI).
Positron emission tomography for bone imaging, as an in vivo tracer technique, allows the measurement of the regional concentration of radioactivity proportional to the image pixel values averaged over a region of interest (ROI) in bones. Positron emission tomography is a functional imaging technique that uses [18F]NaF radiotracer to visualise and quantify regional bone metabolism and blood flow. [18F]NaF has been used for imaging bones for the last 60 years. This article focuses on the pharmacokinetics of [18F]NaF in bones, and various semi-quantitative and quantitative methods for quantifying regional bone metabolism using [18F]NaF PET images.

Julie C. Price is an American medical physicist and professor of radiology at Massachusetts General Hospital (MGH), Harvard Medical School (HMS), as well as the director of PET Pharmacokinetic Modeling at the Athinoula A. Martinos Center at MGH. Price is a leader in the study and application of quantitative positron emission tomography (PET) methods. Prior to this, Price worked with Pittsburgh colleagues to lead the first fully quantitative pharmacokinetic evaluations of 11C-labeled Pittsburgh compound-B (PIB), one of the most widely used PET ligands for imaging amyloid beta plaques. As a principal investigator at MGH, Price continues work to validate novel PET methods for imaging biological markers of health and disease in studies of aging and neurodegeneration, including studies of glucose metabolism, protein expression, neurotransmitter system function, and tau and amyloid beta plaque burden.
References
- ↑ E. J. Hoffman; S.-C. Huang; M. E. Phelps (1979). "Quantitation in positron emission computed tomography: 1. Effect of object size". J. Comput. Assist. Tomogr. 3 (3): 299–308. doi:10.1097/00004728-197906000-00001. PMID 438372. S2CID 39770868.
- ↑ R. M. Kessler; J. R. Ellis, Jr.; M. Eden (1984). "Analysis of emission tomographic scan data: limitations imposed by resolution and background". J. Comput. Assist. Tomogr. 8 (3): 514–522. doi:10.1097/00004728-198406000-00028. PMID 6609942.
- ↑ C. C. Meltzer; J. P. Leal; H. S. Mayberg; H. J. Wagner; J. J. Frost (1990). "Correction of PET data for partial-volume effects in human cerebral cortex by MR imaging". J. Comput. Assist. Tomogr. 14 (4): 561–570. doi:10.1097/00004728-199007000-00011. PMID 2370355. S2CID 27765336.
- ↑ H. W. Müller-Gärtner; J. M. Links; J. L. Prince; R. N. Bryan; E. McVeigh; J. P. Leal; C. Davatzikos; J. J. Frost (1992). "Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects". Journal of Cerebral Blood Flow & Metabolism . 12 (4): 571–583. doi: 10.1038/jcbfm.1992.81 . PMID 1618936.
- ↑ Olivier G. Rousset, Yilong Ma, Alan C. Evans (1998). "Correction for Partial Volume Effects in PET: Principle and Validation". The Journal of Nuclear Medicine . 39 (5): 904–911. PMID 9591599.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Carolyn Cidis Meltzer; Paul E. Kinahan; Phil J. Greer; Thomas E. Nichols; Claude Comtat; Michael N. Cantwell; Michael P. Lin; Julie C. Price (1999). "Comparative Evaluation of MR-Based Partial-Volume Correction Schemes for PET". The Journal of Nuclear Medicine . 40 (12): 2053–2065. PMID 10616886.