A cancer vaccine, or oncovaccine, is a vaccine that either treats existing cancer or prevents development of cancer. Vaccines that treat existing cancer are known as therapeutic cancer vaccines or tumor antigen vaccines. Some of the vaccines are "autologous", being prepared from samples taken from the patient, and are specific to that patient.

Cancer immunotherapy (immuno-oncotherapy) is the stimulation of the immune system to treat cancer, improving the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology (immuno-oncology) and a growing subspecialty of oncology.
An oncolytic virus is a virus that preferentially infects and kills cancer cells. As the infected cancer cells are destroyed by oncolysis, they release new infectious virus particles or virions to help destroy the remaining tumour. Oncolytic viruses are thought not only to cause direct destruction of the tumour cells, but also to stimulate host anti-tumour immune system responses. Oncolytic viruses also have the ability to affect the tumor micro-environment in multiple ways.
Virotherapy is a treatment using biotechnology to convert viruses into therapeutic agents by reprogramming viruses to treat diseases. There are three main branches of virotherapy: anti-cancer oncolytic viruses, viral vectors for gene therapy and viral immunotherapy. These branches use three different types of treatment methods: gene overexpression, gene knockout, and suicide gene delivery. Gene overexpression adds genetic sequences that compensate for low to zero levels of needed gene expression. Gene knockout uses RNA methods to silence or reduce expression of disease-causing genes. Suicide gene delivery introduces genetic sequences that induce an apoptotic response in cells, usually to kill cancerous growths. In a slightly different context, virotherapy can also refer more broadly to the use of viruses to treat certain medical conditions by killing pathogens.

KRAS is a gene that provides instructions for making a protein called K-Ras, a part of the RAS/MAPK pathway. The protein relays signals from outside the cell to the cell's nucleus. These signals instruct the cell to grow and divide (proliferate) or to mature and take on specialized functions (differentiate). It is called KRAS because it was first identified as a viral oncogene in the KirstenRAt Sarcoma virus. The oncogene identified was derived from a cellular genome, so KRAS, when found in a cellular genome, is called a proto-oncogene.
Leronlimab is a humanized monoclonal antibody targeted against the CCR5 receptor found on T lymphocytes of the human immune system. It is being investigated as a potential therapy in the treatment of COVID-19, triple negative breast cancer, and HIV infection. The United States Food and Drug Administration has designated PRO 140 for fast-track approval. In February 2008, the drug entered Phase 2 clinical trials and a phase 3 trial was begun in 2015. In February 2018, Cytodyn Inc reported that the primary endpoint had been achieved in the PRO 140 pivotal combination therapy trial in HIV infection. In 2020 CytoDyn submitted a fast-track biologics license application for treatment of CCR5-tropic HIV-1 Infection.

PARP inhibitors are a group of pharmacological inhibitors of the enzyme poly ADP ribose polymerase (PARP).

Iniparib was a drug candidate for cancer treatment. It was originally believed to act as an irreversible inhibitor of PARP1 and possibly other enzymes through covalent modification, but its effects against PARP were later disproven. It underwent clinical trials for treatment of some types of breast cancer, but was discontinued after disappointing phase III clinical trials.

Veliparib (ABT-888) is a potential anti-cancer drug acting as a PARP inhibitor. It kills cancer cells by blocking a protein called PARP, thereby preventing the repair of DNA or genetic damage in cancer cells and possibly making them more susceptible to anticancer treatments. Veliparib may make whole brain radiation treatment work more effectively against brain metastases from NSCLC. It has been shown to potentiate the effects of many chemotherapeutics, and as such has been part of many combination clinical trials.
Oncolytics Biotech Inc. is a Canadian company headquartered in Calgary, Alberta, that is developing an intravenously delivered immuno-oncolytic virus called pelareorep for the treatment of solid tumors and hematological malignancies. Pelareorep is a non-pathogenic, proprietary isolate of the unmodified reovirus that: induces selective tumor lysis and promotes an inflamed tumor phenotype through innate and adaptive immune responses.
JX-594 is an oncolytic virus is designed to target and destroy cancer cells. It is also known as Pexa-Vec, INN pexastimogene devacirepvec) and was constructed in Dr. Edmund Lattime's lab at Thomas Jefferson University, tested in clinical trials on melanoma patients, and licensed and further developed by SillaJen.

Nivolumab, sold under the brand name Opdivo, is an anti-cancer medication used to treat a number of types of cancer. This includes melanoma, lung cancer, malignant pleural mesothelioma, renal cell carcinoma, Hodgkin lymphoma, head and neck cancer, urothelial carcinoma, colon cancer, esophageal squamous cell carcinoma, liver cancer, gastric cancer, and esophageal or gastroesophageal junction cancer. It is administered intravenously.

Talimogene laherparepvec, sold under the brand name Imlygic among others, is a biopharmaceutical medication used to treat melanoma that cannot be operated on; it is injected directly into a subset of lesions which generates a systemic immune response against the recipient's cancer. The final four year analysis from the pivotal phase 3 study upon which TVEC was approved by the FDA showed a 31.5% response rate with a 16.9% complete response (CR) rate. There was also a substantial and statistically significant survival benefit in patients with earlier metastatic disease and in patients who hadn't received prior systemic treatment for melanoma. The earlier stage group had a reduction in the risk of death of approximately 50% with one in four patients appearing to have met, or be close to be reaching, the medical definition of cure. Real world use of talimogene laherparepvec have shown response rates of up to 88.5% with CR rates of up to 61.5%.

Many variants of herpes simplex virus have been considered for viral therapy of cancer; the early development of these was thoroughly reviewed in the journal Cancer Gene Therapy in 2002. This page describes the most notable variants—those tested in clinical trials: G207, HSV1716, NV1020 and Talimogene laherparepvec. These attenuated versions are constructed by deleting viral genes required for infecting or replicating inside normal cells but not cancer cells, such as ICP34.5, ICP6/UL39, and ICP47.
Adenovirus varieties have been explored extensively as a viral vector for gene therapy and also as an oncolytic virus.
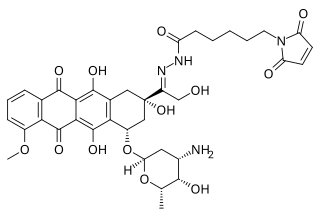
Aldoxorubicin (INNO-206) is a tumor-targeted doxorubicin conjugate in development by CytRx. Specifically, it is the (6-maleimidocaproyl) hydrazone of doxorubicin. Essentially, this chemical name describes doxorubicin attached to an acid-sensitive linker.
GL-ONC1 is an investigational therapeutic product consisting of the clinical grade formulation of the laboratory strain GLV-1h68, an oncolytic virus developed by Genelux Corporation. GL-ONC1 is currently under evaluation in Phase I/II human clinical trials in the United States and Europe.
Viralytics Ltd is an Australian biotechnology company working in the field of oncolytic viruses, that is, viruses that preferentially infect and kill cancer cells. The company's oncolytic virus product, called Cavatak, is currently in clinical trials in metastatic melanoma and other cancers. The drug was granted Orphan Drug status in advanced melanoma in December 2005.

PD-1 inhibitors and PD-L1 inhibitors are a group of checkpoint inhibitor anticancer drugs that block the activity of PD-1 and PDL1 immune checkpoint proteins present on the surface of cells. Immune checkpoint inhibitors are emerging as a front-line treatment for several types of cancer.
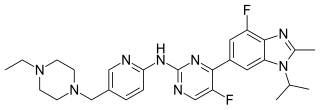
Abemaciclib, sold under the brand name Verzenio among others, is a medication for the treatment of advanced or metastatic breast cancers. It was developed by Eli Lilly and it acts as a CDK inhibitor selective for CDK4 and CDK6.









