In organic chemistry, the phenyl group or phenyl ring is a cyclic group of atoms with the formula C6H5. Phenyl groups are closely related to benzene and can be viewed as a benzene ring, minus a hydrogen, which may be replaced by some other element or compound to serve as a functional group. Phenyl groups have six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the remaining carbon bonded to a substituent. Phenyl groups are commonplace in organic chemistry. Although often depicted with alternating double and single bonds, phenyl groups are chemically aromatic and show nearly equal bond lengths between carbon atoms in the ring.
1,4-Dichlorobenzene (1,4-DCB, p-DCB, or para-dichlorobenzene, sometimes abbreviated as PDB or para) is an organic compound with the formula C6H4Cl2. This colorless solid has a strong odor. The molecule consists of a benzene ring with two chlorine atoms (replacing hydrogen atoms) on opposing sites of the ring.
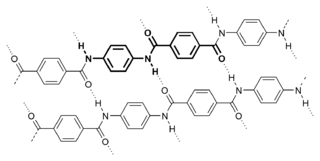
Aramid fibers are a class of heat-resistant and strong synthetic fibers. They are used in aerospace and military applications, for ballistic-rated body armor fabric and ballistic composites, in bicycle tires, marine cordage, marine hull reinforcement, and as an asbestos substitute. The name is a portmanteau of "aromatic polyamide". The chain molecules in the fibers are highly oriented along the fiber axis. As a result, a higher proportion of the chemical bond contributes more to fiber strength than in many other synthetic fibers. Aramides have a very high melting point

p-Phenylenediamine (PPD) is an organic compound with the formula C6H4(NH2)2. This derivative of aniline is a white solid, but samples can darken due to air oxidation. It is mainly used as a component of engineering polymers and composites like kevlar. It is also an ingredient in hair dyes and is occasionally used as a substitute for henna.
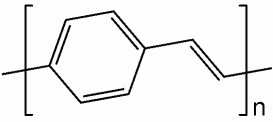
Poly(p-phenylene vinylene) is a conducting polymer of the rigid-rod polymer family. PPV is the only polymer of this type that can be processed into a highly ordered crystalline thin film. PPV and its derivatives are electrically conducting upon doping. Although insoluble in water, its precursors can be manipulated in aqueous solution. The small optical band gap and its bright yellow fluorescence makes PPV a candidate in applications such as light-emitting diodes (LED) and photovoltaic devices. Moreover, PPV can be doped to form electrically conductive materials. Its physical and electronic properties can be altered by the inclusion of functional side groups.
Semi-flexible rod polymers are a kind of organic polymer which may be converted to conductive polymers by appropriate oxidations or doping.

Benzimidazole is a heterocyclic aromatic organic compound. This bicyclic compound consists of the fusion of benzene and imidazole. It is a colorless solid.

o-Phenylenediamine (OPD) is an organic compound with the formula C6H4(NH2)2. This aromatic diamine is an important precursor to many heterocyclic compounds. It is isomeric with m-phenylenediamine and p-phenylenediamine.

m-Phenylenediamine, also called 1,3-diaminobenzene, is an organic compound with the formula C6H4(NH2)2. It is an isomer of o-phenylenediamine and p-phenylenediamine. It is a colourless solid.
Polysulfones are a family of thermoplastic polymers. These polymers are known for their toughness and stability at high temperatures. They contain the subunit aryl-SO2-aryl, the defining feature of which is the sulfone group. Polysulfones were introduced in 1965 by Union Carbide. Due to the high cost of raw materials and processing, polysulfones are used in specialty applications and often are a superior replacement for polycarbonates.
Xylenols are organic compounds with the formula (CH3)2C6H3OH. They are volatile colorless solids or oily liquids. They are derivatives of phenol with two methyl groups and a hydroxyl group. Six isomers exist, of which 2,6-xylenol with both methyl groups in an ortho position with respect to the hydroxyl group is the most important. The name xylenol is a portmanteau of the words xylene and phenol.

2,6-Xylenol is a chemical compound which is one of the six isomers of xylenol. It is also commonly known as 2,6-dimethylphenol (DMP).
A diamine is an organic compound with two amino groups. Diamines are used as monomers to prepare polyamides, polyimides, and polyureas. In terms of quantities produced, 1,6-diaminohexane, a precursor to Nylon 6-6, is most important, followed by ethylenediamine. Vicinal diamines (1,2-diamines) are a structural motif in many biological compounds and are used as ligands in coordination chemistry.

Catecholborane (abbreviated HBcat) is an organoboron compound that is useful in organic synthesis. This colourless liquid is a derivative of catechol and a borane, having the formula C6H4O2BH.

Phosphaalkenes (IUPAC name: alkylidenephosphanes) are organophosphorus compounds with double bonds between carbon and phosphorus(III) with the formula R2C=PR. In the compound phosphorine one carbon atom in benzene is replaced by phosphorus. The reactivity of phosphaalkenes is often compared to that of alkenes and not to that of imines because the HOMO of phosphaalkenes is not the phosphorus lone pair (as in imines the amine lone pair) but the double bond. Therefore like alkenes, phosphaalkenes engage in Wittig reactions, Peterson reactions, Cope rearrangements, and Diels-Alder reactions.
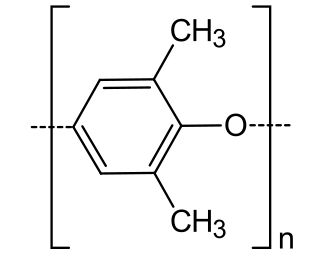
Poly(p-phenylene oxide) or poly(p-phenylene ether) (PPE) is a high-temperature thermoplastic. It is rarely used in its pure form due to difficulties in processing. It is mainly used as blend with polystyrene, high impact styrene-butadiene copolymer or polyamide. PPO is a registered trademark of SABIC Innovative Plastics IP B.V. under which various polyphenylene ether resins are sold.
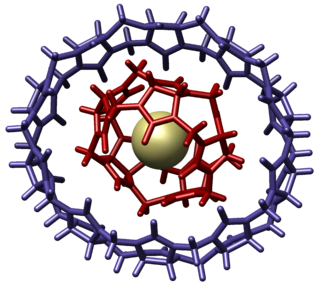
Molecular gyroscopes are chemical compounds or supramolecular complexes containing a rotor that moves freely relative to a stator, and therefore act as gyroscopes. Though any single bond or triple bond permits a chemical group to freely rotate, the compounds described as gyroscopes may protect the rotor from interactions, such as in a crystal structure with low packing density or by physically surrounding the rotor avoiding steric contact. A qualitative distinction can be made based on whether the activation energy needed to overcome rotational barriers is higher than the available thermal energy. If the activation energy required is higher than the available thermal energy, the rotor undergoes "site exchange", jumping in discrete steps between local energy minima on the potential energy surface. If there is thermal energy sufficiently higher than that needed to overcome the barrier to rotation, the molecular rotor can behave more like a macroscopic freely rotating inertial mass.

Cyclobis(paraquat-p-phenylene) belongs to the class of cyclophanes, and consists of aromatic units connected by methylene bridges. It is able to incorporate small guest molecule and has played an important role in host–guest chemistry and supramolecular chemistry.














