See also
| Look up arylene in Wiktionary, the free dictionary. |
An arylene or arenediyl is a substituent of an organic compound that is derived from an aromatic hydrocarbon (arene) and is bivalent, [1] such as phenylene.
| Look up arylene in Wiktionary, the free dictionary. |
An aromatic hydrocarbon or arene is a hydrocarbon with sigma bonds and delocalized pi electrons between carbon atoms forming a circle. In contrast, aliphatic hydrocarbons lack this delocalization. The term "aromatic" was assigned before the physical mechanism determining aromaticity was discovered, and referred simply to the fact that many such compounds have a sweet or pleasant odour; however, not all aromatic compounds have a sweet odour, and not all compounds with a sweet odour are aromatic. The configuration of six carbon atoms in aromatic compounds is called a "benzene ring", after the simplest possible such hydrocarbon, benzene. Aromatic hydrocarbons can be monocyclic (MAH) or polycyclic (PAH).

In organic chemistry, hydrocarbons are divided into two classes: aromatic compounds and aliphatic compounds, also known as non-aromatic hydrocarbons. Aliphatics can be cyclic; however, hydrocarbons with conjugated pi-systems that obey Hückel's rule are instead considered to be aromatic. Aliphatic compounds can be saturated, like hexane, or unsaturated, like hexene and hexyne. Open-chain compounds contain no rings of any type, and are thus aliphatic.

In organic chemistry, functional groups are specific substituents or moieties within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction(s) regardless of the size of the molecule it is a part of. This allows for systematic prediction of chemical reactions and behavior of chemical compounds and design of chemical syntheses. Furthermore, the reactivity of a functional group can be modified by other functional groups nearby. In organic synthesis, functional group interconversion is one of the basic types of transformations.

In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons from which one hydrogen atom has been removed are functional groups called hydrocarbyls. Hydrocarbons are generally colorless and hydrophobic with only weak odors. Because of their diverse molecular structures, it is difficult to generalize further.
Toluene, also known as toluol, is an aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a CH3 group attached to a phenyl group. As such, its IUPAC systematic name is methylbenzene. Toluene is predominantly used as an industrial feedstock and a solvent.

The terpenoids, sometimes called isoprenoids, are a large and diverse class of naturally occurring organic chemicals derived from terpenes. Most are multicyclic structures with oxygen-containing functional groups. About 60% of known natural products are terpenoids. Although sometimes used interchangeably with "terpenes", terpenoids contain additional functional groups, usually O-containing. Terpenes are hydrocarbons.

In organic chemistry, aromaticity is a property of cyclic (ring-shaped), planar (flat) structures with a ring of resonance bonds that gives increased stability compared to other geometric or connective arrangements with the same set of atoms. Aromatic molecules are very stable, and do not break apart easily to react with other substances. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have special stability.

In the context of organic molecules, aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as a placeholder for the aryl group in chemical structure diagrams.

Coronene is a polycyclic aromatic hydrocarbon (PAH) comprising six peri-fused benzene rings. Its chemical formula is C
24H
12. It is a yellow material that dissolves in common solvents including benzene, toluene, and dichloromethane. Its solutions emit blue light fluorescence under UV light. It has been used as a solvent probe, similar to pyrene.
Polycyclic aromatic hydrocarbons are hydrocarbons—organic compounds containing only carbon and hydrogen—that are composed of multiple aromatic rings. The simplest such chemicals are naphthalene, having two aromatic rings, and the three-ring compounds anthracene and phenanthrene.

Annulenes are monocyclic hydrocarbons that contain the maximum number of non-cumulated double bonds ('mancude'). They have the general formula CnHn or CnHn+1. The IUPAC naming conventions are that annulenes with 7 or more carbon atoms are named as [n]annulene, where n is the number of carbon atoms in their ring, though sometimes the smaller annulenes are referred to using the same notation, and benzene is sometimes referred to simply as annulene.
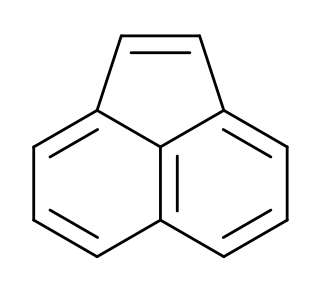
Acenaphthylene, a polycyclic aromatic hydrocarbon is an ortho- and peri-fused tricyclic hydrocarbon. The molecule resembles naphthalene with positions 1 and 8 connected by a -CH=CH- unit. It is a yellow solid. Unlike many polycyclic aromatic hydrocarbons, it has no fluorescence.
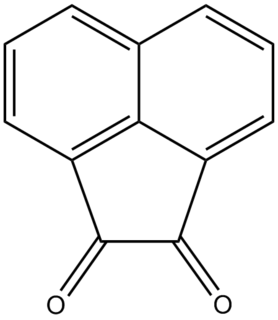
Acenaphthoquinone is a quinone derived from acenaphthene. It is a water-insoluble yellow solid. It is a precursor to some agrichemicals and dyes.

Picene is a hydrocarbon found in the pitchy residue obtained in the distillation of peat tar and of petroleum. This is distilled to dryness and the distillate repeatedly recrystallized from cymene. It may be synthetically prepared by the action of anhydrous aluminium chloride on a mixture of naphthalene and 1,2-dibromoethane, or by distilling a-dinaphthostilbene. It crystallizes in large colorless plates which possess a blue fluorescence. It is soluble in concentrated sulfuric acid with a green color. Chromic acid in glacial acetic acid solution oxidizes it to picene-quinone, picene-quinone carboxylic acid, and finally to phthalic acid.

Fluoranthene is a polycyclic aromatic hydrocarbon (PAH). The molecule can be viewed as the fusion of naphthalene and benzene unit connected by a five-membered ring. Although samples are often pale yellow, the compound is colorless. It is soluble in nonpolar organic solvents. It is a member of the class of PAHs known as non-alternant PAHs because it has rings other than those with six carbon atoms. It is a structural isomer of the alternant PAH pyrene. It is not as thermodynamically stable as pyrene. Its name is derived from its fluorescence under UV light.

Hydrazines (R2N-NR2) are a class of chemical compounds which have two nitrogen atoms linked via a covalent bond and which carry from one up to four alkyl or aryl substituents. Hydrazines can be considered as derivatives of the inorganic hydrazine (H2N-NH2), in which one or more hydrogen atoms have been replaced by hydrocarbon groups.

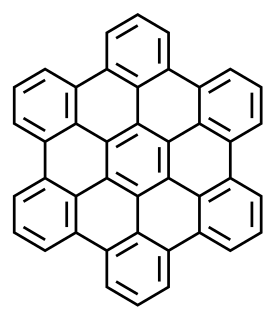
Ovalene is a polycyclic aromatic hydrocarbon with the formula C32H14, which consists of ten peri-fused six-membered rings. It is very similar to coronene.

Benz[a]anthracene or benzo[a]anthracene is a polycyclic aromatic hydrocarbon with the chemical formula C18H12. It is produced during incomplete combustion of organic matter.

Benzo[a]fluorene is a polycyclic aromatic hydrocarbon (PAH). It is currently listed as a Group 3 carcinogen by the IARC.

The von Baeyer nomenclature is a system for describing polycyclic hydrocarbons. The system was originally developed in 1900 by Adolf von Baeyer for bicyclic systems and in 1913 expanded by Eduard Buchner and Wilhelm Weigand for tricyclic systems. The system has been adopted and extended by the IUPAC as part of its nomenclature for organic chemistry. The modern version has been extended to cover more cases of compounds including an arbitrary number of cycles, heterocyclic compounds and unsaturated compounds.
| This article about an aromatic compound is a stub. You can help Wikipedia by expanding it. |