Phosphoglycerate may refer to:
| This set index page lists chemical compounds articles associated with the same name. If an internal link led you here, you may wish to change the link to point directly to the intended article. |
Phosphoglycerate may refer to:
| This set index page lists chemical compounds articles associated with the same name. If an internal link led you here, you may wish to change the link to point directly to the intended article. |

Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO−, and a hydrogen ion, H+. The free energy released in this process is used to form the high-energy molecules ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide). Glycolysis is a sequence of ten enzyme-catalyzed reactions. Most monosaccharides, such as fructose and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful rather than just utilized as steps in the overall reaction. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat.
Serine is an ɑ-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group, a carboxyl group, and a side chain consisting of a hydroxymethyl group, classifying it as a polar amino acid. It can be synthesized in the human body under normal physiological circumstances, making it a nonessential amino acid. It is encoded by the codons UCU, UCC, UCA, UCG, AGU and AGC.
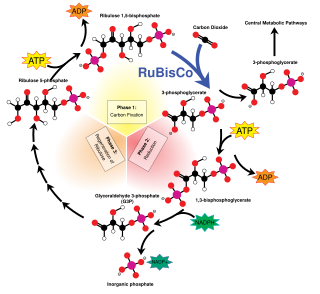
C3 carbon fixation is the most common of three metabolic pathways for carbon fixation in photosynthesis, along with C4 and CAM. This process converts carbon dioxide and ribulose bisphosphate (RuBP, a 5-carbon sugar) into two molecules of 3-phosphoglycerate through the following reaction:
3-Phosphoglyceric acid (3PG) is the conjugate acid of glycerate 3-phosphate (GP). The glycerate is a biochemically significant metabolic intermediate in both glycolysis and the Calvin cycle. This anion is often termed as PGA when referring to the Calvin cycle. In the Calvin cycle, 3-phosphoglycerate is the product of the spontaneous scission of an unstable 6-carbon intermediate formed upon CO2 fixation. Thus, two equivalents of 3-phosphoglycerate are produced for each molecule of CO2 that is fixed.

1,3-Bisphosphoglyceric acid (1,3-Bisphosphoglycerate or 1,3BPG) is a 3-carbon organic molecule present in most, if not all, living organisms. It primarily exists as a metabolic intermediate in both glycolysis during respiration and the Calvin cycle during photosynthesis. 1,3BPG is a transitional stage between glycerate 3-phosphate and glyceraldehyde 3-phosphate during the fixation/reduction of CO2. 1,3BPG is also a precursor to 2,3-bisphosphoglycerate which in turn is a reaction intermediate in the glycolytic pathway.
A mutase is an enzyme of the isomerase class that catalyzes the movement of a functional group from one position to another within the same molecule. In other words, mutases catalyze intramolecular group transfers. Examples of mutases include bisphosphoglycerate mutase, which appears in red blood cells and phosphoglycerate mutase, which is an enzyme integral to glycolysis. In glycolysis, it changes 3-phosphoglycerate to 2-phosphoglycerate. In particular it moves phosphate groups within a single molecule, for instance: phosphoglycerate mutase.
The complete glucose breakdown is a series of chemical reactions representing transformation of glucose to adenosine triphosphate during the normal phases of aerobic cellular respiration. It is mostly done inside the mitochondria to release the maximum amount of energy.

2,3-Bisphosphoglyceric acid (2,3-BPG), also known as 2,3-diphosphoglyceric acid (2,3-DPG), is a three-carbon isomer of the glycolytic intermediate 1,3-bisphosphoglyceric acid (1,3-BPG). 2,3-BPG is present in human red blood cells at approximately 5 mmol/L. It binds with greater affinity to deoxygenated hemoglobin than it does to oxygenated hemoglobin due to conformational differences: 2,3-BPG fits in the deoxygenated hemoglobin conformation, but not as well in the oxygenated conformation. It interacts with deoxygenated hemoglobin beta subunits and so it decreases the affinity for oxygen and allosterically promotes the release of the remaining oxygen molecules bound to the hemoglobin; therefore it enhances the ability of RBCs to release oxygen near tissues that need it most. 2,3-BPG is thus an allosteric effector.

2-Phosphoglyceric acid (2PG), or 2-phosphoglycerate, is a glyceric acid which serves as the substrate in the ninth step of glycolysis. It is catalyzed by enolase into phosphoenolpyruvate (PEP), the penultimate step in the conversion of glucose to pyruvate.

Phosphoglycerate kinase is an enzyme that catalyzes the reversible transfer of a phosphate group from 1,3-bisphosphoglycerate (1,3-BPG) to ADP producing 3-phosphoglycerate (3-PG) and ATP :

Phosphoglycerate mutase (PGM) is any enzyme that catalyzes step 8 of glycolysis. They catalyze the internal transfer of a phosphate group from C-3 to C-2 which results in the conversion of 3-phosphoglycerate (3PG) to 2-phosphoglycerate (2PG) through a 2,3-bisphosphoglycerate intermediate. These enzymes are categorized into the two distinct classes of either cofactor-dependent (dPGM) or cofactor-independent (iPGM). The dPGM enzyme is composed of approximately 250 amino acids and is found in all vertebrates as well as in some invertebrates, fungi, and bacteria. The iPGM class is found in all plants and algae as well as in some invertebrate, fungi, and Gram-positive bacteria. This class of PGM enzyme shares the same superfamily as alkaline phosphatase.
Glucose-1,6-bisphosphate synthase is a type of enzyme called a phosphotransferase and is involved in mammalian starch and sucrose metabolism. It catalyzes the transfer of a phosphate group from 1,3-bisphosphoglycerate to glucose-1-phosphate, yielding 3-phosphoglycerate and glucose-1,6-bisphosphate.

Phosphoglycerate kinase 1 is an enzyme that in humans is encoded by the PGK1 gene.
In enzymology, a 3-phosphoglycerate phosphatase (EC 3.1.3.38) is an enzyme that catalyzes the chemical reaction
In enzymology, an ADP-phosphoglycerate phosphatase (EC 3.1.3.28) is an enzyme that catalyzes the chemical reaction
In enzymology, a mannosyl-3-phosphoglycerate phosphatase (EC 3.1.3.70) is an enzyme that catalyzes the chemical reaction
In enzymology, a mannosyl-3-phosphoglycerate synthase is an enzyme that catalyzes the chemical reaction

In enzymology, D-3-phosphoglycerate dehydrogenase (PHGDH) (EC 1.1.1.95) is an enzyme that primarily catalyzes the chemical reactions

Phosphoglycerate mutase 2 (PGAM2), also known as muscle-specific phosphoglycerate mutase (PGAM-M), is a phosphoglycerate mutase that, in humans, is encoded by the PGAM2 gene on chromosome 7.
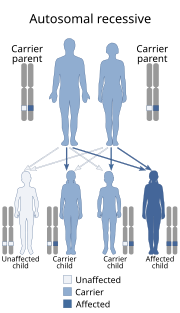
D-glycerate dehydrogenase deficiency or PHGDH is a rare autosomal metabolic disease where the young patient is unable to produce an enzyme necessary to convert 3-phosphoglycerate into 3-phosphohydroxypyruvate, which is the only way for humans to synthesize serine.