Related Research Articles

Allotropy or allotropism is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: the atoms of the element are bonded together in a different manner. For example, the allotropes of carbon include diamond, graphite, graphene, and fullerenes.

Melting, or fusion, is a physical process that results in the phase transition of a substance from a solid to a liquid. This occurs when the internal energy of the solid increases, typically by the application of heat or pressure, which increases the substance's temperature to the melting point. At the melting point, the ordering of ions or molecules in the solid breaks down to a less ordered state, and the solid "melts" to become a liquid.

Piezoelectricity is the electric charge that accumulates in certain solid materials—such as crystals, certain ceramics, and biological matter such as bone, DNA, and various proteins—in response to applied mechanical stress. The word piezoelectricity means electricity resulting from pressure and latent heat. It is derived from the Greek word πιέζειν; piezein, which means to squeeze or press, and ἤλεκτρον ēlektron, which means amber, an ancient source of electric charge.
An electro–optic effect is a change in the optical properties of a material in response to an electric field that varies slowly compared with the frequency of light. The term encompasses a number of distinct phenomena, which can be subdivided into
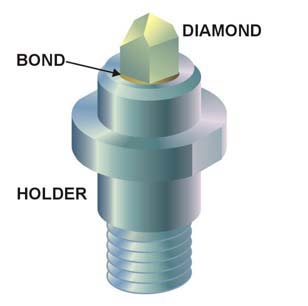
A superhard material is a material with a hardness value exceeding 40 gigapascals (GPa) when measured by the Vickers hardness test. They are virtually incompressible solids with high electron density and high bond covalency. As a result of their unique properties, these materials are of great interest in many industrial areas including, but not limited to, abrasives, polishing and cutting tools, disc brakes, and wear-resistant and protective coatings.

Superconductivity is the phenomenon of certain materials exhibiting zero electrical resistance and the expulsion of magnetic fields below a characteristic temperature. The history of superconductivity began with Dutch physicist Heike Kamerlingh Onnes's discovery of superconductivity in mercury in 1911. Since then, many other superconducting materials have been discovered and the theory of superconductivity has been developed. These subjects remain active areas of study in the field of condensed matter physics.

Lithium niobate is a non-naturally-occurring salt consisting of niobium, lithium, and oxygen. Its single crystals are an important material for optical waveguides, mobile phones, piezoelectric sensors, optical modulators and various other linear and non-linear optical applications. Lithium niobate is sometimes referred to by the brand name linobate.

A piezoelectric sensor is a device that uses the piezoelectric effect to measure changes in pressure, acceleration, temperature, strain, or force by converting them to an electrical charge. The prefix piezo- is Greek for 'press' or 'squeeze'.
The piezoresistive effect is a change in the electrical resistivity of a semiconductor or metal when mechanical strain is applied. In contrast to the piezoelectric effect, the piezoresistive effect causes a change only in electrical resistance, not in electric potential.
In physics, photon-induced electric field poling is a phenomenon whereby a pattern of local electric field orientations can be encoded in a suitable ferroelectric material, such as perovskite. The resulting encoded material is conceptually similar to the pattern of magnetic field orientations within the magnetic domains of a ferromagnet, and thus may be considered as a possible technology for computer storage media. The encoded regions are optically active and thus may be "read out" optically.
Covalent organic frameworks (COFs) are a class of materials that form two- or three- dimensional structures through reactions between organic precursors resulting in strong, covalent bonds to afford porous, stable, and crystalline materials. COFs emerged as a field from the overarching domain of organic materials as researchers optimized both synthetic control and precursor selection. These improvements to coordination chemistry enabled non-porous and amorphous organic materials such as organic polymers to advance into the construction of porous, crystalline materials with rigid structures that granted exceptional material stability in a wide range of solvents and conditions. Through the development of reticular chemistry, precise synthetic control was achieved and resulted in ordered, nano-porous structures with highly preferential structural orientation and properties which could be synergistically enhanced and amplified. With judicious selection of COF secondary building units (SBUs), or precursors, the final structure could be predetermined, and modified with exceptional control enabling fine-tuning of emergent properties. This level of control facilitates the COF material to be designed, synthesized, and utilized in various applications, many times with metrics on scale or surpassing that of the current state-of-the-art approaches.
Organic photorefractive materials are materials that exhibit a temporary change in refractive index when exposed to light. The changing refractive index causes light to change speed throughout the material and produce light and dark regions in the crystal. The buildup can be controlled to produce holographic images for use in biomedical scans and optical computing. The ease with which the chemical composition can be changed in organic materials makes the photorefractive effect more controllable.
Lithium molybdenum purple bronze is a chemical compound with formula Li
0.9Mo
6O
17, that is, a mixed oxide of molybdenum and lithium. It can be obtained as flat crystals with a purple-red color and metallic sheen.

Solid nitrogen is a number of solid forms of the element nitrogen, first observed in 1884. Solid nitrogen is mainly the subject of academic research, but low-temperature, low-pressure solid nitrogen is a substantial component of bodies in the outer Solar System and high-temperature, high-pressure solid nitrogen is a powerful explosive, with higher energy density than any other non-nuclear material.
A polar metal, metallic ferroelectric, or ferroelectric metal is a metal that contains an electric dipole moment. Its components have an ordered electric dipole. Such metals should be unexpected, because the charge should conduct by way of the free electrons in the metal and neutralize the polarized charge. However they do exist. Probably the first report of a polar metal was in single crystals of the cuprate superconductors YBa2Cu3O7−δ,. A polarization was observed along one (001) axis by pyroelectric effect measurements, and the sign of the polarization was shown to be reversible, while its magnitude could be increased by poling with an electric field. The polarization was found to disappear in the superconducting state. The lattice distortions responsible were considered to be a result of oxygen ion displacements induced by doped charges that break inversion symmetry. The effect was utilized for fabrication of pyroelectric detectors for space applications, having the advantage of large pyroelectric coefficient and low intrinsic resistance. Another substance family that can produce a polar metal is the nickelate perovskites. One example interpreted to show polar metallic behavior is lanthanum nickelate, LaNiO3. A thin film of LaNiO3 grown on the (111) crystal face of lanthanum aluminate, (LaAlO3) was interpreted to be both conductor and a polar material at room temperature. The resistivity of this system, however, shows an upturn with decreasing temperature, hence does not strictly adhere to the definition of a metal. Also, when grown 3 or 4 unit cells thick (1-2 nm) on the (100) crystal face of LaAlO3, the LaNiO3 can be a polar insulator or polar metal depending on the atomic termination of the surface. Lithium osmate, LiOsO3 also undergoes a ferrorelectric transition when it is cooled below 140K. The point group changes from R3c to R3c losing its centrosymmetry. At room temperature and below, lithium osmate is an electric conductor, in single crystal, polycrystalline or powder forms, and the ferroelectric form only appears below 140K. Above 140K the material behaves like a normal metal. Artificial two-dimensional polar metal by charge transfer to a ferroelectric insulator has been realized in LaAlO3/Ba0.8Sr0.2TiO3/SrTiO3 complex oxide heterostructures.

Kenji Uchino is an American electronics engineer, physicist, academic, inventor and industry executive. He is currently a Professor of Electrical Engineering at Pennsylvania State University, where he also directs the International Center for Actuators and Transducers at Materials Research Institute. He is the former Associate Director at The US Office of Naval Research – Global Tokyo Office.
Selenogallates are chemical compounds which contain anionic units of selenium connected to gallium. They can be considered as gallates where selenium substitutes for oxygen. Similar compounds include the thiogallates and selenostannates. They are in the category of chalcogenotrielates or more broadly chalcogenometallates.
Corundum is the name for a structure prototype in inorganic solids, derived from the namesake polymorph of aluminum oxide (α-Al2O3). Other compounds, especially among the inorganic solids, exist in corundum structure, either in ambient or other conditions. Corundum structures are associated with metal-insulator transition, ferroelectricity, polar magnetism, and magnetoelectric effects.
References
- ↑ Vedam, K.; Limsuwan, Pichet (13 October 1975). "Piezo-optic Behavior of Water and Carbon Tetrachloride under High Pressure". Physical Review Letters. American Physical Society (APS). 35 (15): 1014–1016. doi:10.1103/physrevlett.35.1014. ISSN 0031-9007.
- ↑ Sveleba, S. A.; Polovinko, I. I.; Bublyk, M. I.; Kapustianik, V. B.; Krochuk, A. S. (1 September 1995). "Peculiarities of the Piezooptic Effect in Incommensurate Crystals". Physica Status Solidi B. Wiley. 191 (1): 227–234. doi:10.1002/pssb.2221910124. ISSN 0370-1972.
- ↑ Mytsyk, Bogdan G.; Andrushchak, Anatoliy S.; Demyanyshyn, Nataliya M.; Kost', Yaroslav P.; Kityk, Andriy V.; Mandracci, Pietro; Schranz, Wilfried (25 March 2009). "Piezo-optic coefficients of MgO-doped LiNbO3 crystals". Applied Optics. The Optical Society. 48 (10): 1904–1911. doi:10.1364/ao.48.001904. ISSN 0003-6935. PMID 19340145.
- ↑ Bain, A. K.; Rao, K. Veerabhadra; Chand, Prem; Yamaguchi, T.; Wada, M. (21 October 2008). "Piezooptic Dispersion of Li2Ge7015 Crystals". Ferroelectrics. Informa UK Limited. 366 (1): 16–21. doi:10.1080/00150190802363074. ISSN 0015-0193. S2CID 120106390.