Related Research Articles

A carbon nanotube (CNT) is a tube made of carbon with a diameter in the nanometer range (nanoscale). They are one of the allotropes of carbon.
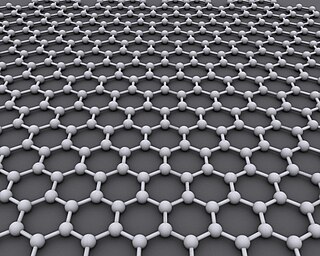
Graphene is an allotrope of carbon consisting of a single layer of atoms arranged in a hexagonal lattice nanostructure. The name is derived from "graphite" and the suffix -ene, reflecting the fact that the graphite allotrope of carbon contains numerous double bonds.

James Mitchell Tour is an American chemist and nanotechnologist. He is a Professor of Chemistry, Professor of Materials Science and Nanoengineering, and Professor of Computer Science at Rice University in Houston, Texas.

Several methods exist for storing hydrogen. These include mechanical approaches such as using high pressures and low temperatures, or employing chemical compounds that release H2 upon demand. While large amounts of hydrogen are produced by various industries, it is mostly consumed at the site of production, notably for the synthesis of ammonia. For many years hydrogen has been stored as compressed gas or cryogenic liquid, and transported as such in cylinders, tubes, and cryogenic tanks for use in industry or as propellant in space programs. Interest in using hydrogen for on-board storage of energy in zero-emissions vehicles is motivating the development of new methods of storage, more adapted to this new application. The overarching challenge is the very low boiling point of H2: it boils around 20.268 K (−252.882 °C or −423.188 °F). Achieving such low temperatures requires expending significant energy.

Carbon nanotubes (CNTs) are cylinders of one or more layers of graphene (lattice). Diameters of single-walled carbon nanotubes (SWNTs) and multi-walled carbon nanotubes (MWNTs) are typically 0.8 to 2 nm and 5 to 20 nm, respectively, although MWNT diameters can exceed 100 nm. CNT lengths range from less than 100 nm to 0.5 m.

Nanobatteries are fabricated batteries employing technology at the nanoscale, particles that measure less than 100 nanometers or 10−7 meters. These batteries may be nano in size or may use nanotechnology in a macro scale battery. Nanoscale batteries can be combined to function as a macrobattery such as within a nanopore battery.
As the world's energy demand continues to grow, the development of more efficient and sustainable technologies for generating and storing energy is becoming increasingly important. According to Dr. Wade Adams from Rice University, energy will be the most pressing problem facing humanity in the next 50 years and nanotechnology has potential to solve this issue. Nanotechnology, a relatively new field of science and engineering, has shown promise to have a significant impact on the energy industry. Nanotechnology is defined as any technology that contains particles with one dimension under 100 nanometers in length. For scale, a single virus particle is about 100 nanometers wide.

Graphite oxide (GO), formerly called graphitic oxide or graphitic acid, is a compound of carbon, oxygen, and hydrogen in variable ratios, obtained by treating graphite with strong oxidizers and acids for resolving of extra metals. The maximally oxidized bulk product is a yellow solid with C:O ratio between 2.1 and 2.9, that retains the layer structure of graphite but with a much larger and irregular spacing.
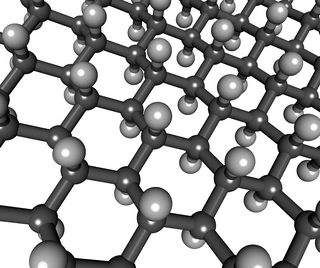
Graphane is a two-dimensional polymer of carbon and hydrogen with the formula unit (CH)n where n is large. Partial hydrogenation results in hydrogenated graphene, which was reported by Elias et al in 2009 by a TEM study to be "direct evidence for a new graphene-based derivative". The authors viewed the panorama as "a whole range of new two-dimensional crystals with designed electronic and other properties".
Carbide-derived carbon (CDC), also known as tunable nanoporous carbon, is the common term for carbon materials derived from carbide precursors, such as binary (e.g. SiC, TiC), or ternary carbides, also known as MAX phases (e.g., Ti2AlC, Ti3SiC2). CDCs have also been derived from polymer-derived ceramics such as Si-O-C or Ti-C, and carbonitrides, such as Si-N-C. CDCs can occur in various structures, ranging from amorphous to crystalline carbon, from sp2- to sp3-bonded, and from highly porous to fully dense. Among others, the following carbon structures have been derived from carbide precursors: micro- and mesoporous carbon, amorphous carbon, carbon nanotubes, onion-like carbon, nanocrystalline diamond, graphene, and graphite. Among carbon materials, microporous CDCs exhibit some of the highest reported specific surface areas (up to more than 3000 m2/g). By varying the type of the precursor and the CDC synthesis conditions, microporous and mesoporous structures with controllable average pore size and pore size distributions can be produced. Depending on the precursor and the synthesis conditions, the average pore size control can be applied at sub-Angstrom accuracy. This ability to precisely tune the size and shapes of pores makes CDCs attractive for selective sorption and storage of liquids and gases (e.g., hydrogen, methane, CO2) and the high electric conductivity and electrochemical stability allows these structures to be effectively implemented in electrical energy storage and capacitive water desalinization.

Single-walled carbon nanohorn is the name given by Sumio Iijima and colleagues in 1999 to horn-shaped sheath aggregate of graphene sheets. Very similar structures had been observed in 1994 by Peter J.F. Harris, Edman Tsang, John Claridge and Malcolm Green. Ever since the discovery of the fullerene, the family of carbon nanostructures has been steadily expanded. Included in this family are single-walled and multi-walled carbon nanotubes, carbon onions and cones and, most recently, SWNHs. These SWNHs with about 40–50 nm in tubule length and about 2–3 nm in diameter are derived from SWNTs and ended by a five-pentagon conical cap with a cone opening angle of ~20o. Moreover, thousands of SWNHs associate with each other to form the ‘dahlia-like' and ‘bud-like’ structured aggregates which have an average diameter of about 80–100 nm. The former consists of tubules and graphene sheets protruding from its surface like petals of a dahlia, while the latter is composed of tubules developing inside the particle itself. Their unique structures with high surface area and microporosity make SWNHs become a promising material for gas adsorption, biosensing, drug delivery, gas storage and catalyst support for fuel cell. Single-walled carbon nanohorns are an example of the family of carbon nanocones.
Potential graphene applications include lightweight, thin, and flexible electric/photonics circuits, solar cells, and various medical, chemical and industrial processes enhanced or enabled by the use of new graphene materials.
In materials science, the term single-layer materials or 2D materials refers to crystalline solids consisting of a single layer of atoms. These materials are promising for some applications but remain the focus of research. Single-layer materials derived from single elements generally carry the -ene suffix in their names, e.g. graphene. Single-layer materials that are compounds of two or more elements have -ane or -ide suffixes. 2D materials can generally be categorized as either 2D allotropes of various elements or as compounds.
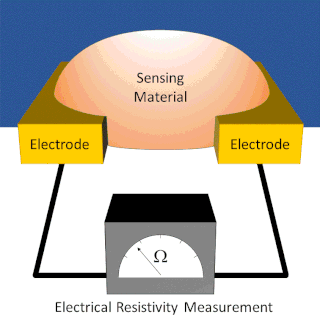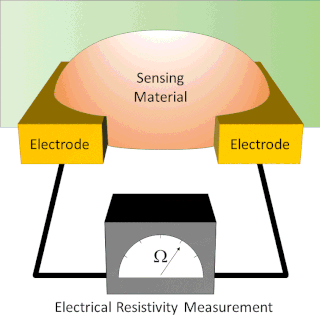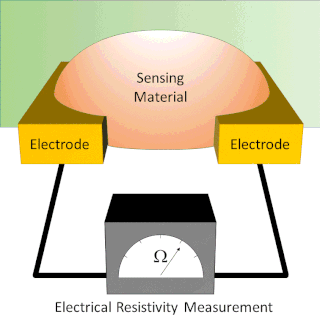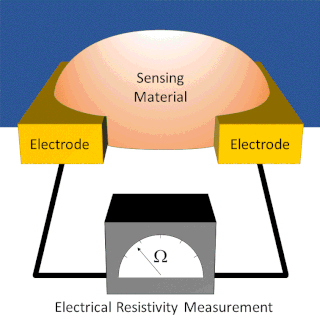
A chemiresistor is a material that changes its electrical resistance in response to changes in the nearby chemical environment. Chemiresistors are a class of chemical sensors that rely on the direct chemical interaction between the sensing material and the analyte. The sensing material and the analyte can interact by covalent bonding, hydrogen bonding, or molecular recognition. Several different materials have chemiresistor properties: metal-oxide semiconductors, some conductive polymers, and nanomaterials like graphene, carbon nanotubes and nanoparticles. Typically these materials are used as partially selective sensors in devices like electronic tongues or electronic noses.

In materials and electric battery research, cobalt oxide nanoparticles usually refers to particles of cobalt(II,III) oxide Co
3O
4 of nanometer size, with various shapes and crystal structures.
A rapidly increasing list of graphene production techniques have been developed to enable graphene's use in commercial applications.
Graphene-Boron Nitride nanohybrid materials are a class of compounds created from graphene and boron nitride nanosheets. Graphene and boron nitride both contain intrinsic thermally conductive and electrically insulative properties. The combination of these two compounds may be useful to advance the development and understanding of electronics.
A graphene morphology is any of the structures related to, and formed from, single sheets of graphene. 'Graphene' is typically used to refer to the crystalline monolayer of the naturally occurring material graphite. Due to quantum confinement of electrons within the material at these low dimensions, small differences in graphene morphology can greatly impact the physical and chemical properties of these materials. Commonly studied graphene morphologies include the monolayer sheets, bilayer sheets, graphene nanoribbons and other 3D structures formed from stacking of the monolayer sheets.
A graphene helix, similar to the carbon nanotube, is a structure consisting of a two-dimensional sheet of graphene wrapped into a helix. These graphene sheets can have multiple layers, called multi-walled carbon structures, that add to these helices thus increasing their tensile strength but increasing the difficulty of manufacturing. Using van der Waals interactions it can make structures within one another.
Petra Rudolf is a German and Italian solid state physicist. As of 2003, Rudolf has been a professor at the Materials Science Centre, University of Groningen, Netherlands.
References
- ↑ GK Dimitrakakis; et al. (2008). "Pillared Graphene: A New 3-D Network Nanostructure for Enhanced Hydrogen Storage". Nano Letters. 8 (10): 3166–3170. Bibcode:2008NanoL...8.3166D. doi:10.1021/nl801417w. PMID 18800853.