The fertility factor allows genes to be transferred from one bacterium carrying the factor to another bacterium lacking the factor by conjugation. The F factor is carried on the F episome, the first episome to be discovered. Unlike other plasmids, F factor is constitutive for transfer proteins due to a mutation in the gene finO. The F plasmid belongs to a class of conjugative plasmids that control sexual functions of bacteria with a fertility inhibition (Fin) system.
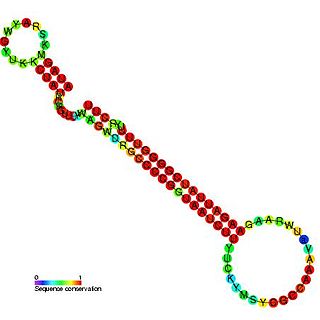
CopA-like RNA is a family of non-coding RNAs found on the R1 plasmid.
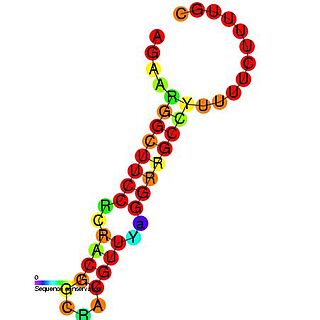
In molecular biology ctRNA is a plasmid encoded noncoding RNA that binds to the mRNA of repB and causes translational inhibition. ctRNA is encoded by plasmids and functions in rolling circle replication to maintain a low copy number. In Corynebacterium glutamicum, it achieves this by antisense pairing with the mRNA of RepB, a replication initiation protein. In Enterococcus faecium the plasmid pJB01 contains three open reading frames, copA, repB, and repC. The pJB01 ctRNA is coded on the opposite strand from the copA/repB intergenic region and partially overlaps an atypical ribosome binding site for repB.

FinP encodes an antisense non-coding RNA gene that is complementary to part of the TraJ 5' UTR. The FinOP system regulates the transfer of F-like plasmids. The traJ gene encodes a protein required for transcription from the major transfer promoter, pY. The FinO protein is essential for effective repression, acting by binding to FinP and protecting it from RNase E degradation.

The micF RNA is a non-coding RNA stress response gene found in Escherichia coli and related bacteria that post-transcriptionally controls expression of the outer membrane porin gene ompF. The micF gene encodes a non-translated 93 nucleotide antisense RNA that binds its target ompF mRNA and regulates ompF expression by inhibiting translation and inducing degradation of the message. In addition, other factors, such as the RNA chaperone protein StpA also play a role in this regulatory system. Expression of micF is controlled by both environmental and internal stress factors. Four transcriptional regulators are known to bind the micF promoter region and activate micF expression.
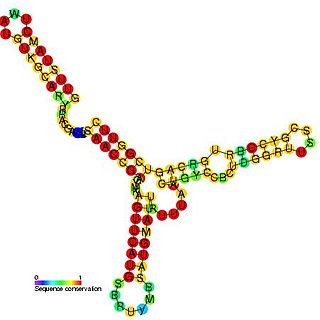
Anti-Q RNA is a small ncRNA from the conjugal plasmid pCF10 of Enterococcus faecalis. It is coded in cis to its regulatory target, prgQ, but can also act in trans. Anti-Q is known to interact with nascent prgQ transcripts to allow formation of an intrinsic terminator, or attenuator, thus preventing transcription of downstream genes. This mode of regulation is essentially the same as that of the countertranscript-driven attenuators that control copy number in pT181, pAMbeta1 and pIP501 and related Staphylococcal plasmids.
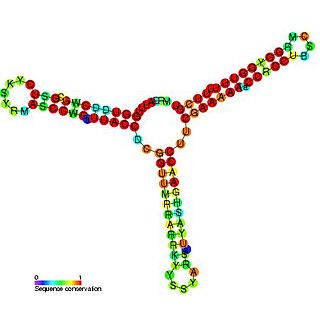
RNAI is a non-coding RNA that is an antisense repressor of the replication of some E. coli plasmids, including ColE1. Plasmid replication is usually initiated by RNAII, which acts as a primer by binding to its template DNA. The complementary RNAI binds RNAII prohibiting it from its initiation role. The rate of degradation of RNAI is therefore a major factor in the control of plasmid replication. This rate of degradation is aided by the pcnB gene product, which polyadenylates the 3' end of RNAI targeting it for degradation by PNPase.
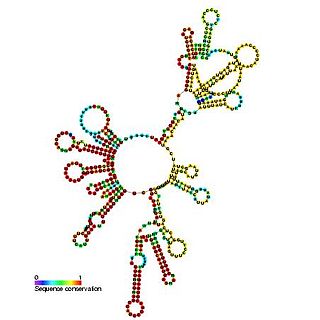
RNAIII is a small RNA which is known to regulate the expression of many Staphylococcus aureus genes encoding exoproteins and cell wall associated proteins. In S. aureus, RNAIII acts as the effector of the agr quorum sensing system and is transcribed from the P3 operon. The RNAIII transcript also contains the 26 amino acid delta-haemolysin gene (hld). RNAIII regulates the expression of the transcription factor rot by blocking its translation. It has been suggested that RNAIII binds to the rot mRNA in an antisense fashion occluding the Shine-Dalgarno sequence.
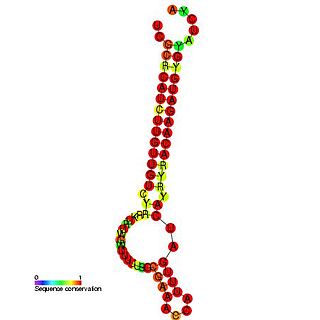
RNA-OUT is a non-coding RNA that is antisense to the RNA-IN non-coding RNA. Transposition of insertion sequence IS10 is regulated by an anti-sense RNA which inhibits transposase expression when IS10 is present in multiple copies per cell. IS10 antisense pairing is facilitated by the RNA-binding protein, Hfq. RNA-OUT consists of a stem-loop domain topped by a flexibly paired loop; the 5' end of the target molecule, RNA-IN, is complementary to the top of the loop, and complementarity extends for 35 nucleotides down one side of RNA-OUT.
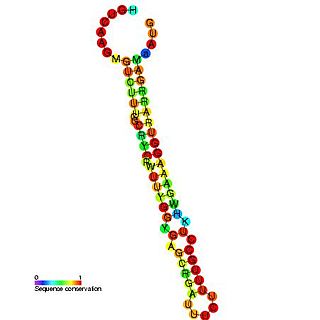
The S-element is an RNA element found in p42d and related plasmids. The S-element has multiple functions and is believed to act as a negative regulator of repC transcription, and be required for efficient replication and act as a translational enhancer of repC.

The traJ 5' UTR is a cis acting RNA element which is involved in regulating plasmid transfer in bacteria.
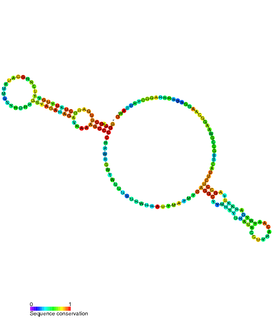
The hok/sok system is a postsegregational killing mechanism employed by the R1 plasmid in Escherichia coli. It was the first type I toxin-antitoxin pair to be identified through characterisation of a plasmid-stabilising locus. It is a type I system because the toxin is neutralised by a complementary RNA, rather than a partnered protein.

6C RNA is a class of non-coding RNA present in actinomycetes. 6C RNA was originally discovered as a conserved RNA structure having two stem-loops each containing six or more cytosine (C) residues. Later work revealed that 6C RNAs in Streptomyces coelicolor and Streptomyces avermitilis have predicted rho-independent transcription terminators, and microarray and reverse-transcriptase PCR experiments indicate that the S. coelicolor version is transcribed as RNA. Transcription of the S. coelicolor RNA increases during sporulation, and three transcripts were detected that overlap the 6C motif, but have different apparent start and stop sites.

The Bacillus-plasmid RNA motif is a predicted conserved RNA structure usually located in plasmids. It is known in species under the genera Bacillus and Lactobacillus. In Bacillus subtilis, it is found upstream of the hypothetical gene ydcS, whose function is unknown.

The traJ-II RNA motif is a conserved RNA structure discovered in bacteria by using bioinformatics. traJ-II RNAs appear to be in the 5' untranslated regions of protein-coding genes called traJ, which functions in the process of bacterial conjugation. A previously identified motif known as TraJ 5' UTR is also found upstream of traJ genes functions as the target of FinP antisense RNAs, so it is possible that traJ-II RNAs play a similar role as targets of an antisense RNA. However, some sequence features within the traJ-II RNA motif suggest that the biological RNA might be transcribed from the reverse-complement strand. Thus is it unclear whether traJ-II function as cis-regulatory elements. traJ-II RNAs are found in a variety of proteobacteria.
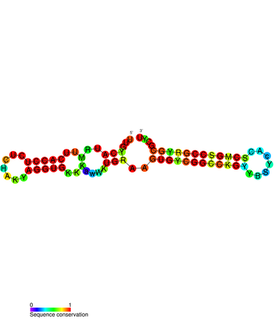
PtaRNA1 is a family of non-coding RNAs. Homologs of PtaRNA1 can be found in the proteobacteria families, Betaproteobacteria and Gammaproteobacteria. In all cases the PtaRNA1 is located anti-sense to a short protein-coding gene. In Xanthomonas campestris pv. vesicatoria, this gene is annotated as XCV2162 and is included in the plasmid toxin family of proteins.
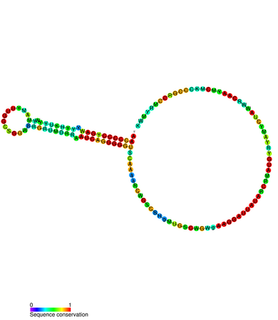
In plasmids, the regulatory region of repBA gene forms a pseudoknot. The repA gene, which encodes a protein likely to function as an initiator for replication, and the repB gene are translationally coupled. The leader sequence of the repA mRNA contains two complementary sequences of 8 bases. Base-pairing between these two sequences forms a pseudoknot which is essential for translation. The first of these complementary sequences is found within a stem-loop, which forms a target for RNAI. Binding of RNAI to this stem-loop inhibits pseudoknot formation and translation of RepA.

The FlmA-FlmB toxin-antitoxin system consists of FlmB RNA, a family of non-coding RNAs and the protein toxin FlmA. The FlmB RNA transcript is 100 nucleotides in length and is homologous to sok RNA from the hok/sok system and fulfills the identical function as a post-segregational killing (PSK) mechanism.

The par stability determinant is a 400 bp locus of the pAD1 plasmid which encodes a type I toxin-antitoxin system in Enterococcus faecalis. It was the first such plasmid addiction module to be found in gram-positive bacteria.
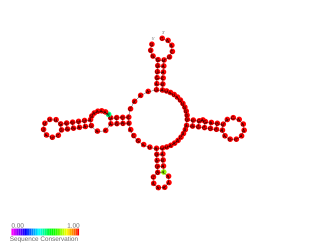
RnaG is a small regulatory non-coding RNA encoded by the virulence plasmid of Shigella flexneri, a Gram-negative pathogenic bacterium that causes human bacillary dysentery. It is a first regulatory RNA characterised in S. flexneri. The RNA is 450 nucleotides long and it contains a region with specific secondary structure that interacts with icsA mRNA and forms a transcription terminator. Acting as antisense, RnaG is transcribed from the complementary strand of its target, icsA mRNA. The activity of the incA protein is crucial for spreading of the bacterial pathogen in the host cells.






















